+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Bacterial serine protease | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Seine protease / HYDROLASE | |||||||||
| Function / homology |  Function and homology information Function and homology informationpeptidase Do / cell envelope / : / periplasmic space / serine-type endopeptidase activity / identical protein binding Similarity search - Function | |||||||||
| Biological species |   | |||||||||
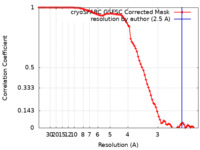
| Method | single particle reconstruction / cryo EM / Resolution: 2.5 Å | |||||||||
 Authors Authors | Lee I-G / Jeon H | |||||||||
| Funding support |  Korea, Republic Of, 1 items Korea, Republic Of, 1 items
| |||||||||
 Citation Citation |  Journal: Adv Mater / Year: 2024 Journal: Adv Mater / Year: 2024Title: Polymorphic Self-Assembly with Procedural Flexibility for Monodisperse Quaternary Protein Structures of DegQ Enzymes. Authors: Hanul Jeon / Ah-Reum Han / Sangmin Oh / Jin-Gyeong Park / Myeong Namkoong / Kyeong-Mi Bang / Ho Min Kim / Nak-Kyoon Kim / Kwang Yeon Hwang / Kahyun Hur / Bong-Jin Lee / Jeongyun Heo / Sehoon ...Authors: Hanul Jeon / Ah-Reum Han / Sangmin Oh / Jin-Gyeong Park / Myeong Namkoong / Kyeong-Mi Bang / Ho Min Kim / Nak-Kyoon Kim / Kwang Yeon Hwang / Kahyun Hur / Bong-Jin Lee / Jeongyun Heo / Sehoon Kim / Hyun Kyu Song / Hyesung Cho / In-Gyun Lee /  Abstract: As large molecular tertiary structures, some proteins can act as small robots that find, bind, and chaperone target protein clients, showing the potential to serve as smart building blocks in self- ...As large molecular tertiary structures, some proteins can act as small robots that find, bind, and chaperone target protein clients, showing the potential to serve as smart building blocks in self-assembly fields. Instead of using such intrinsic functions, most self-assembly methodologies for proteins aim for de novo-designed structures with accurate geometric assemblies, which can limit procedural flexibility. Here, a strategy enabling polymorphic clustering of quaternary proteins, exhibiting simplicity and flexibility of self-assembling paths for proteins in forming monodisperse quaternary cage particles is presented. It is proposed that the enzyme protomer DegQ, previously solved at low resolution, may potentially be usable as a threefold symmetric building block, which can form polyhedral cages incorporated by the chaperone action of DegQ in the presence of protein clients. To obtain highly monodisperse cage particles, soft, and hence, less resistive client proteins, which can program the inherent chaperone activity of DegQ to efficient formations of polymorphic cages, depending on the size of clients are utilized. By reconstructing the atomic resolution cryogenic electron microscopy DegQ structures using obtained 12- and 24-meric clusters, the polymorphic clustering of DegQ enzymes is validated in terms of soft and rigid domains, which will provide effective routes for protein self-assemblies with procedural flexibility. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_37257.map.gz emd_37257.map.gz | 89.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-37257-v30.xml emd-37257-v30.xml emd-37257.xml emd-37257.xml | 22.5 KB 22.5 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_37257_fsc.xml emd_37257_fsc.xml | 8.9 KB | Display |  FSC data file FSC data file |
| Images |  emd_37257.png emd_37257.png | 55.6 KB | ||
| Filedesc metadata |  emd-37257.cif.gz emd-37257.cif.gz | 6.4 KB | ||
| Others |  emd_37257_half_map_1.map.gz emd_37257_half_map_1.map.gz emd_37257_half_map_2.map.gz emd_37257_half_map_2.map.gz | 165.4 MB 165.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-37257 http://ftp.pdbj.org/pub/emdb/structures/EMD-37257 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-37257 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-37257 | HTTPS FTP |
-Related structure data
| Related structure data |  8kicMC  8w69C C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_37257.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_37257.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.848 Å | ||||||||||||||||||||||||||||||||||||
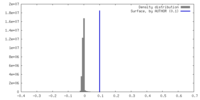
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_37257_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
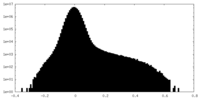
| Projections & Slices |
| ||||||||||||
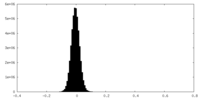
| Density Histograms |
-Half map: #1
| File | emd_37257_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
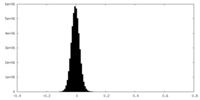
| Density Histograms |
- Sample components
Sample components
-Entire : Bacterial serine protease-lysozyme complex
| Entire | Name: Bacterial serine protease-lysozyme complex |
|---|---|
| Components |
|
-Supramolecule #1: Bacterial serine protease-lysozyme complex
| Supramolecule | Name: Bacterial serine protease-lysozyme complex / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: peptidase Do
| Macromolecule | Name: peptidase Do / type: protein_or_peptide / ID: 1 / Number of copies: 6 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 48.361047 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MKKQTQLLSA LALSVGLTLS ASFQAVASIP GQVADQAPLP SLAPMLEKVL PAVVSVRVEG TASQGQKIPE EFKKFFGDDL PDQPAQPFE GLGSGVIINA SKGYVLTNNH VINQAQKISI QLNDGREFDA KLIGSDDQSD IALLQIQNPS KLTQIAIADS D KLRVGDFA ...String: MKKQTQLLSA LALSVGLTLS ASFQAVASIP GQVADQAPLP SLAPMLEKVL PAVVSVRVEG TASQGQKIPE EFKKFFGDDL PDQPAQPFE GLGSGVIINA SKGYVLTNNH VINQAQKISI QLNDGREFDA KLIGSDDQSD IALLQIQNPS KLTQIAIADS D KLRVGDFA VAVGNPFGLG QTATSGIVSA LGRSGLNLEG LENFIQTDAS INRGNAGGAL LNLNGELIGI NTAILAPGGG SV GIGFAIP SNMARTLAQQ LIDFGEIKRG LLGIKGTEMS ADIAKAFNLD VQRGAFVSEV LPGSGSAKAG VKAGDIITSL NGK PLNSFA ELRSRIATTE PGTKVKLGLL RNGKPLEVEV TLDTSTSSSA SAEMITPALE GATLSDGQLK DGGKGIKIDE VVKG SPAAQ AGLQKDDVII GVNRDRVNSI AEMRKVLAAK PAIIALQIVR GNESIYLLMR LWHHHHHH UniProtKB: peptidase Do |
-Macromolecule #2: Lysozyme fragment (unknown sequence)
| Macromolecule | Name: Lysozyme fragment (unknown sequence) / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 698.854 Da |
| Sequence | String: (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) |
-Macromolecule #3: Lysozyme fragment (unknown sequence)
| Macromolecule | Name: Lysozyme fragment (unknown sequence) / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 443.539 Da |
| Sequence | String: (UNK)(UNK)(UNK)(UNK)(UNK) |
-Macromolecule #4: Lysozyme fragment (unknown sequence)
| Macromolecule | Name: Lysozyme fragment (unknown sequence) / type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 613.749 Da |
| Sequence | String: (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | TFS KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 67.7 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 1.7 µm / Nominal defocus min: 0.7000000000000001 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller







 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)