[English] 日本語
 Yorodumi
Yorodumi- EMDB-36888: Cryo-EM structure of Kaposi's Sarcoma-Associated Herpesvirus-G Pr... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of Kaposi's Sarcoma-Associated Herpesvirus-G Protein-Coupled Receptor (KSHV-GPCR)in complex with CXC chemokine CXCL1 | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Kaposi's Sarcoma Herpesvirus GPCR / KSHV-GPCR / chemokine / VIRAL PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationC-C chemokine receptor activity / C-C chemokine binding / chemokine activity / Chemokine receptors bind chemokines / Interleukin-10 signaling / G protein-coupled serotonin receptor binding / cell chemotaxis / growth factor activity / enzyme activator activity / calcium-mediated signaling ...C-C chemokine receptor activity / C-C chemokine binding / chemokine activity / Chemokine receptors bind chemokines / Interleukin-10 signaling / G protein-coupled serotonin receptor binding / cell chemotaxis / growth factor activity / enzyme activator activity / calcium-mediated signaling / adenylate cyclase-modulating G protein-coupled receptor signaling pathway / specific granule lumen / G-protein beta/gamma-subunit complex binding / Olfactory Signaling Pathway / Activation of the phototransduction cascade / chemotaxis / G beta:gamma signalling through PLC beta / Presynaptic function of Kainate receptors / Thromboxane signalling through TP receptor / G protein-coupled acetylcholine receptor signaling pathway / Activation of G protein gated Potassium channels / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / G-protein activation / G beta:gamma signalling through CDC42 / Prostacyclin signalling through prostacyclin receptor / Glucagon signaling in metabolic regulation / G beta:gamma signalling through BTK / Synthesis, secretion, and inactivation of Glucagon-like Peptide-1 (GLP-1) / ADP signalling through P2Y purinoceptor 12 / photoreceptor disc membrane / Sensory perception of sweet, bitter, and umami (glutamate) taste / Glucagon-type ligand receptors / Adrenaline,noradrenaline inhibits insulin secretion / Vasopressin regulates renal water homeostasis via Aquaporins / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / G alpha (z) signalling events / tertiary granule lumen / ADP signalling through P2Y purinoceptor 1 / cellular response to catecholamine stimulus / ADORA2B mediated anti-inflammatory cytokines production / G beta:gamma signalling through PI3Kgamma / adenylate cyclase-activating dopamine receptor signaling pathway / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / nervous system development / GPER1 signaling / G-protein beta-subunit binding / cellular response to prostaglandin E stimulus / heterotrimeric G-protein complex / Inactivation, recovery and regulation of the phototransduction cascade / G alpha (12/13) signalling events / extracellular vesicle / sensory perception of taste / Thrombin signalling through proteinase activated receptors (PARs) / signaling receptor complex adaptor activity / positive regulation of cytosolic calcium ion concentration / retina development in camera-type eye / actin cytoskeleton organization / GTPase binding / Ca2+ pathway / cell cortex / High laminar flow shear stress activates signaling by PIEZO1 and PECAM1:CDH5:KDR in endothelial cells / G alpha (i) signalling events / G alpha (s) signalling events / phospholipase C-activating G protein-coupled receptor signaling pathway / G alpha (q) signalling events / Ras protein signal transduction / Extra-nuclear estrogen signaling / cell population proliferation / intracellular signal transduction / immune response / G protein-coupled receptor signaling pathway / inflammatory response / signaling receptor binding / negative regulation of cell population proliferation / lysosomal membrane / cell division / GTPase activity / synapse / Neutrophil degranulation / centrosome / GTP binding / protein-containing complex binding / signal transduction / extracellular space / extracellular exosome / extracellular region / metal ion binding / nucleus / membrane / plasma membrane / cytosol / cytoplasm Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) /   Human gammaherpesvirus 8 Human gammaherpesvirus 8 | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.01 Å | |||||||||
 Authors Authors | Liu YZ / Liu AJ | |||||||||
| Funding support | 1 items
| |||||||||
 Citation Citation |  Journal: Proc Natl Acad Sci U S A / Year: 2024 Journal: Proc Natl Acad Sci U S A / Year: 2024Title: Structural insights into KSHV-GPCR constitutive activation and CXCL1 chemokine recognition. Authors: Aijun Liu / Yezhou Liu / Clàudia Llinàs Del Torrent Masachs / Weijia Zhang / Leonardo Pardo / Richard D Ye /   Abstract: Kaposi's sarcoma-associated herpesvirus (KSHV) encodes a viral G protein-coupled receptor, KSHV-GPCR, that contributes to KSHV immune evasion and pathogenesis of Kaposi's sarcoma. KSHV-GPCR shares a ...Kaposi's sarcoma-associated herpesvirus (KSHV) encodes a viral G protein-coupled receptor, KSHV-GPCR, that contributes to KSHV immune evasion and pathogenesis of Kaposi's sarcoma. KSHV-GPCR shares a high similarity with CXC chemokine receptors CXCR2 and can be activated by selected chemokine ligands. Like other herpesvirus-encoded GPCRs, KSHV-GPCR is characterized by its constitutive activity by coupling to various G proteins. We investigated the structural basis of ligand-dependent and constitutive activation of KSHV-GPCR, obtaining high-resolution cryo-EM structures of KSHV-GPCR-Gi complexes with and without the bound CXCL1 chemokine. Analysis of the apo-KSHV-GPCR-Gi structure (2.81 Å) unraveled the involvement of extracellular loop 2 in constitutive activation of the receptor. In comparison, the CXCL1-bound KSHV-GPCR-Gi structure (3.01 Å) showed a two-site binding mode and provided detailed information of CXCL1 binding to a chemokine receptor. The dual activation mechanism employed by KSHV-GPCR represents an evolutionary adaptation for immune evasion and contributes to the pathogenesis of Kaposi's sarcoma. Together with results from functional assays that confirmed the structural models, these findings may help to develop therapeutic strategies for KSHV infection. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_36888.map.gz emd_36888.map.gz | 117.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-36888-v30.xml emd-36888-v30.xml emd-36888.xml emd-36888.xml | 18.6 KB 18.6 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_36888.png emd_36888.png | 43.6 KB | ||
| Filedesc metadata |  emd-36888.cif.gz emd-36888.cif.gz | 6.2 KB | ||
| Others |  emd_36888_half_map_1.map.gz emd_36888_half_map_1.map.gz emd_36888_half_map_2.map.gz emd_36888_half_map_2.map.gz | 116.1 MB 116.1 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-36888 http://ftp.pdbj.org/pub/emdb/structures/EMD-36888 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-36888 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-36888 | HTTPS FTP |
-Related structure data
| Related structure data |  8k4oMC  8k4pC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_36888.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_36888.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.85 Å | ||||||||||||||||||||||||||||||||||||
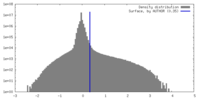
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #1
| File | emd_36888_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_36888_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Kaposi's Sarcoma-Associated Herpesvirus-G Protein-Coupled Recepto...
| Entire | Name: Kaposi's Sarcoma-Associated Herpesvirus-G Protein-Coupled Receptor (KSHV-GPCR)in complex with CXC chemokine CXCL1 and Gi protein |
|---|---|
| Components |
|
-Supramolecule #1: Kaposi's Sarcoma-Associated Herpesvirus-G Protein-Coupled Recepto...
| Supramolecule | Name: Kaposi's Sarcoma-Associated Herpesvirus-G Protein-Coupled Receptor (KSHV-GPCR)in complex with CXC chemokine CXCL1 and Gi protein type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: G protein-coupled receptor
| Macromolecule | Name: G protein-coupled receptor / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Human gammaherpesvirus 8 Human gammaherpesvirus 8 |
| Molecular weight | Theoretical: 37.881773 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: DFLTIFLDDD ESWNETLNMS GYDYSGNFSL EVSVCEMTTV VPYTWNVGIL SLIFLINVLG NGLVTYIFCK HRSRAGAIDI LLLGICLNS LCLSISLLAE VLMFLFPNII STGLCRLEIF FYYLYVYLDI FSVVCVSLVR YLLVAYSTRS WPKKQSLGWV L TSAALLIA ...String: DFLTIFLDDD ESWNETLNMS GYDYSGNFSL EVSVCEMTTV VPYTWNVGIL SLIFLINVLG NGLVTYIFCK HRSRAGAIDI LLLGICLNS LCLSISLLAE VLMFLFPNII STGLCRLEIF FYYLYVYLDI FSVVCVSLVR YLLVAYSTRS WPKKQSLGWV L TSAALLIA LVLSGDACRH RSRVVDPVSK QAMCYENAGN MTADWRLHVR TVSVTAGFLL PLALLILFYA LTWCVVRRTK LQ ARRKVRG VIVAVVLLFF VFCFPYHVLN LLDTLLRRRW IRDSCYTRGL INVGLAVTSL LQALYSAVVP LIYSCLGSLF RQR MYGLFQ SLRQSFM UniProtKB: G protein-coupled receptor |
-Macromolecule #2: Growth-regulated alpha protein
| Macromolecule | Name: Growth-regulated alpha protein / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 6.727939 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: ASVATELRCQ CLQTLQGIHP KNIQSVNVKS PGPHCAQTEV IATLKNGRKA CLNPASPIVK KII UniProtKB: Growth-regulated alpha protein |
-Macromolecule #3: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 36.956383 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: DQLRQEAEQL KNQIRDARKA CADATLSQIT NNIDPVGRIQ MRTRRTLRGH LAKIYAMHWG TDSRLLVSAS QDGKLIIWDS YTTNKVHAI PLRSSWVMTC AYAPSGNYVA CGGLDNICSI YNLKTREGNV RVSRELAGHT GYLSCCRFLD DNQIVTSSGD T TCALWDIE ...String: DQLRQEAEQL KNQIRDARKA CADATLSQIT NNIDPVGRIQ MRTRRTLRGH LAKIYAMHWG TDSRLLVSAS QDGKLIIWDS YTTNKVHAI PLRSSWVMTC AYAPSGNYVA CGGLDNICSI YNLKTREGNV RVSRELAGHT GYLSCCRFLD DNQIVTSSGD T TCALWDIE TGQQTTTFTG HTGDVMSLSL APDTRLFVSG ACDASAKLWD VREGMCRQTF TGHESDINAI CFFPNGNAFA TG SDDATCR LFDLRADQEL MTYSHDNIIC GITSVSFSKS GRLLLAGYDD FNCNVWDALK ADRAGVLAGH DNRVSCLGVT DDG MAVATG SWDSFLKIWN UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 |
-Macromolecule #4: Guanine nucleotide-binding protein G(I) subunit alpha-1
| Macromolecule | Name: Guanine nucleotide-binding protein G(I) subunit alpha-1 type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 39.914406 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: LSAEDKAAVE RSKMIDRNLR EDGEKAAREV KLLLLGAGES GKSTIVKQMK IIHEAGYSEE ECKQYKAVVY SNTIQSIIAI IRAMGRLKI DFGDAARADD ARQLFVLAGS AEEGFMTAEL AGVIKRLWKD GGVQACFSRS REYQLNDSAA YYLNDLDRIS Q GSYIPTQQ ...String: LSAEDKAAVE RSKMIDRNLR EDGEKAAREV KLLLLGAGES GKSTIVKQMK IIHEAGYSEE ECKQYKAVVY SNTIQSIIAI IRAMGRLKI DFGDAARADD ARQLFVLAGS AEEGFMTAEL AGVIKRLWKD GGVQACFSRS REYQLNDSAA YYLNDLDRIS Q GSYIPTQQ DVLRTRVKTT GIVETHFTFK DLHFKMFDVG GQRSERKKWI HCFEGVTAII FCVALSDYDL VLAEDEEMNR MH ESMKLFD SICNNKWFTD TSIILFLNKK DLFEEKIKKS PLTICYPEYA GSNTYEEAAA YIQCQFEDLN KRKDTKEIYT HFT CATDTK NVQFVFDAVT DVIIKNNLKD CGLF UniProtKB: Guanine nucleotide-binding protein G(i) subunit alpha-1 |
-Macromolecule #5: Guanine nucleotide-binding protein subunit gamma
| Macromolecule | Name: Guanine nucleotide-binding protein subunit gamma / type: protein_or_peptide / ID: 5 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 6.00197 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: IAQARKLVEQ LKMEANIDRI KVSKAAADLM AYCEAHAKED PLLTPVPASE NPFR UniProtKB: Guanine nucleotide-binding protein subunit gamma |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Grid | Material: GOLD |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.5 µm / Nominal defocus min: 1.2 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: PDB ENTRY PDB model - PDB ID: |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.01 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 107180 |
| Initial angle assignment | Type: NOT APPLICABLE |
| Final angle assignment | Type: NOT APPLICABLE |
 Movie
Movie Controller
Controller



























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)