+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Short ago complexed with TIR-APAZ | ||||||||||||||||||
 Map data Map data | |||||||||||||||||||
 Sample Sample |
| ||||||||||||||||||
 Keywords Keywords | SPARTA / Ago / Tir / DNA BINDING PROTEIN | ||||||||||||||||||
| Function / homology | TIR domain / Toll/interleukin-1 receptor homology (TIR) domain / Toll/interleukin-1 receptor homology (TIR) domain superfamily / Ribonuclease H superfamily / nucleic acid binding / Ribonuclease H-like superfamily / signal transduction / Piwi domain-containing protein / TIR domain-containing protein Function and homology information Function and homology information | ||||||||||||||||||
| Biological species |  Thermoflavifilum thermophilum (bacteria) Thermoflavifilum thermophilum (bacteria) | ||||||||||||||||||
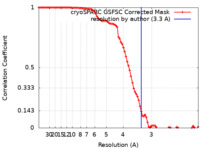
| Method | single particle reconstruction / cryo EM / Resolution: 3.3 Å | ||||||||||||||||||
 Authors Authors | Guo LJ / Huang PP / Li ZX / Xiao YB / Chen MR | ||||||||||||||||||
| Funding support |  China, 5 items China, 5 items
| ||||||||||||||||||
 Citation Citation |  Journal: Nat Chem Biol / Year: 2024 Journal: Nat Chem Biol / Year: 2024Title: Auto-inhibition and activation of a short Argonaute-associated TIR-APAZ defense system. Authors: Lijie Guo / Pingping Huang / Zhaoxing Li / Young-Cheul Shin / Purui Yan / Meiling Lu / Meirong Chen / Yibei Xiao /  Abstract: Short prokaryotic Ago accounts for most prokaryotic Argonaute proteins (pAgos) and is involved in defending bacteria against invading nucleic acids. Short pAgo associated with TIR-APAZ (SPARTA) has ...Short prokaryotic Ago accounts for most prokaryotic Argonaute proteins (pAgos) and is involved in defending bacteria against invading nucleic acids. Short pAgo associated with TIR-APAZ (SPARTA) has been shown to oligomerize and deplete NAD upon guide-mediated target DNA recognition. However, the molecular basis of SPARTA inhibition and activation remains unknown. In this study, we determined the cryogenic electron microscopy structures of Crenotalea thermophila SPARTA in its inhibited, transient and activated states. The SPARTA monomer is auto-inhibited by its acidic tail, which occupies the guide-target binding channel. Guide-mediated target binding expels this acidic tail and triggers substantial conformational changes to expose the Ago-Ago dimerization interface. As a result, SPARTA assembles into an active tetramer, where the four TIR domains are rearranged and packed to form NADase active sites. Together with biochemical evidence, our results provide a panoramic vision explaining SPARTA auto-inhibition and activation and expand understanding of pAgo-mediated bacterial defense systems. | ||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_36059.map.gz emd_36059.map.gz | 32.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-36059-v30.xml emd-36059-v30.xml emd-36059.xml emd-36059.xml | 16.5 KB 16.5 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_36059_fsc.xml emd_36059_fsc.xml | 9 KB | Display |  FSC data file FSC data file |
| Images |  emd_36059.png emd_36059.png | 33.9 KB | ||
| Filedesc metadata |  emd-36059.cif.gz emd-36059.cif.gz | 6 KB | ||
| Others |  emd_36059_half_map_1.map.gz emd_36059_half_map_1.map.gz emd_36059_half_map_2.map.gz emd_36059_half_map_2.map.gz | 59.4 MB 59.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-36059 http://ftp.pdbj.org/pub/emdb/structures/EMD-36059 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-36059 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-36059 | HTTPS FTP |
-Validation report
| Summary document |  emd_36059_validation.pdf.gz emd_36059_validation.pdf.gz | 875.6 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_36059_full_validation.pdf.gz emd_36059_full_validation.pdf.gz | 875.2 KB | Display | |
| Data in XML |  emd_36059_validation.xml.gz emd_36059_validation.xml.gz | 16.5 KB | Display | |
| Data in CIF |  emd_36059_validation.cif.gz emd_36059_validation.cif.gz | 21.3 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-36059 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-36059 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-36059 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-36059 | HTTPS FTP |
-Related structure data
| Related structure data |  8j84MC  8j8hC  8j9gC  8j9pC  8jayC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_36059.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_36059.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.05 Å | ||||||||||||||||||||||||||||||||||||
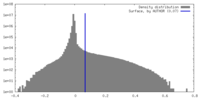
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_36059_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
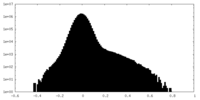
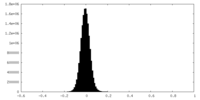
| Density Histograms |
-Half map: #1
| File | emd_36059_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
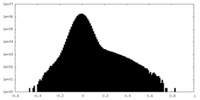
| Density Histograms |
- Sample components
Sample components
-Entire : Short ago complexed with TIR-APAZ
| Entire | Name: Short ago complexed with TIR-APAZ |
|---|---|
| Components |
|
-Supramolecule #1: Short ago complexed with TIR-APAZ
| Supramolecule | Name: Short ago complexed with TIR-APAZ / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Thermoflavifilum thermophilum (bacteria) Thermoflavifilum thermophilum (bacteria) |
-Macromolecule #1: Piwi domain-containing protein
| Macromolecule | Name: Piwi domain-containing protein / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Thermoflavifilum thermophilum (bacteria) Thermoflavifilum thermophilum (bacteria) |
| Molecular weight | Theoretical: 58.304848 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MKELIYIEEP SILFAHGQKC TDPRDGLALF GPLNQIYGIK SGVVGTQKGL QIFKSYLDKI QKPIYNHNNI TRPMFPGFEA VFGCKWESQ NIVFKEITDE EIRRYLFNAS THKRTYDLVT LFNDKIITAN KNDEERVDVW FVIVPEEIYK YCRPNSVLPN E LVQTKSLI ...String: MKELIYIEEP SILFAHGQKC TDPRDGLALF GPLNQIYGIK SGVVGTQKGL QIFKSYLDKI QKPIYNHNNI TRPMFPGFEA VFGCKWESQ NIVFKEITDE EIRRYLFNAS THKRTYDLVT LFNDKIITAN KNDEERVDVW FVIVPEEIYK YCRPNSVLPN E LVQTKSLI SKSKAKSFRY TPTLFEEFNK KLKEVEKEAK TYNYDAQFHD QLKARLLEHT IPTQILREST LAWRDFKNTF GA PIRDFSK IEGHLAWTIS TAAYYKAGGK PWKLGDIRPG VCYLGLVYKK IEKSKNPQNA CCAAQMFLDN GDGTVFKGEV GPW YNPEKG EYHLKPKEAK ALLTQALESY KEQNKSYPKE VFIHARTRFN DEEWNAFNEV TPKNTNLVGV TITKSKPLKL YKTE GAFPI MRGNAYIVDE KKAFLWTLGF VPKLQSTLSM EVPNPIFIEI NKGEAEIQQV LKDILALTKL NYNACIYADG EPVTL RFAN KIGEILTAST EIKTPPLAFK YYI UniProtKB: Piwi domain-containing protein |
-Macromolecule #2: TIR domain-containing protein
| Macromolecule | Name: TIR domain-containing protein / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Thermoflavifilum thermophilum (bacteria) Thermoflavifilum thermophilum (bacteria) |
| Molecular weight | Theoretical: 53.256734 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MRNKIFISHA TPEDDDFTRW LSLKLIGLGY EVWCDILFLD KGVDFWSTIE KEIRENTCKF LIVSSTAGNK REGVLKELAV ATKVKKHLQ DDMFIIPLAI DENLSYDDIN IEIVRLNAID FKKSWAKGLQ DLLDAFEKQN VPKKPPDHSK SNLLYQQIFL H DKQAIEKE ...String: MRNKIFISHA TPEDDDFTRW LSLKLIGLGY EVWCDILFLD KGVDFWSTIE KEIRENTCKF LIVSSTAGNK REGVLKELAV ATKVKKHLQ DDMFIIPLAI DENLSYDDIN IEIVRLNAID FKKSWAKGLQ DLLDAFEKQN VPKKPPDHSK SNLLYQQIFL H DKQAIEKE ETYDSNWFPI ISFPNELRFH RYDWRLPKQF DVRTLAFPAI RYKEYLCTFA WEYDFIHQLP KTETYNGQES IR ISTSDIL SGRYDTDFIR NYECQRLIVQ LINKAFELRM KDKNVREYQM SKTFAYWIEK GKLEKDKFEK IKLVGKQKNK YWH FGISAA GKLYPSPVLM VSSHIIFTMD GINLIKSKSI QHSSRRKQGK NWWNDKWREK LLAFIRFLSD DQNAIYLNVG SEEK ILISN KPLKFFGKMS YVTPSEVTLE EESVLADINN FEEDTEDLDE LEDIE UniProtKB: TIR domain-containing protein |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Grid | Model: Quantifoil R1.2/1.3 / Material: GOLD |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Average electron dose: 40.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.4 µm / Nominal defocus min: 1.0 µm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: AB INITIO MODEL |
|---|---|
| Output model |  PDB-8j84: |
 Movie
Movie Controller
Controller









 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)