+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| タイトル | human KCNQ2-CaM-Ebio1 complex in the presence of PIP2 | |||||||||
 マップデータ マップデータ | ||||||||||
 試料 試料 |
| |||||||||
 キーワード キーワード | Potassium voltage-gated channel subfamily KQT member 2 / Ebio1 / MEMBRANE PROTEIN | |||||||||
| 機能・相同性 |  機能・相同性情報 機能・相同性情報axon initial segment / Voltage gated Potassium channels / node of Ranvier / voltage-gated monoatomic cation channel activity / CaM pathway / Cam-PDE 1 activation / Interaction between L1 and Ankyrins / Sodium/Calcium exchangers / Calmodulin induced events / Reduction of cytosolic Ca++ levels ...axon initial segment / Voltage gated Potassium channels / node of Ranvier / voltage-gated monoatomic cation channel activity / CaM pathway / Cam-PDE 1 activation / Interaction between L1 and Ankyrins / Sodium/Calcium exchangers / Calmodulin induced events / Reduction of cytosolic Ca++ levels / Activation of Ca-permeable Kainate Receptor / CREB1 phosphorylation through the activation of CaMKII/CaMKK/CaMKIV cascasde / Loss of phosphorylation of MECP2 at T308 / ankyrin binding / CREB1 phosphorylation through the activation of Adenylate Cyclase / PKA activation / negative regulation of high voltage-gated calcium channel activity / CaMK IV-mediated phosphorylation of CREB / Glycogen breakdown (glycogenolysis) / organelle localization by membrane tethering / negative regulation of calcium ion export across plasma membrane / CLEC7A (Dectin-1) induces NFAT activation / Activation of RAC1 downstream of NMDARs / mitochondrion-endoplasmic reticulum membrane tethering / autophagosome membrane docking / presynaptic endocytosis / regulation of cardiac muscle cell action potential / negative regulation of peptidyl-threonine phosphorylation / calcineurin-mediated signaling / positive regulation of ryanodine-sensitive calcium-release channel activity / regulation of cell communication by electrical coupling involved in cardiac conduction / Synthesis of IP3 and IP4 in the cytosol / negative regulation of ryanodine-sensitive calcium-release channel activity / Phase 0 - rapid depolarisation / Negative regulation of NMDA receptor-mediated neuronal transmission / Unblocking of NMDA receptors, glutamate binding and activation / RHO GTPases activate PAKs / Ion transport by P-type ATPases / Uptake and function of anthrax toxins / protein phosphatase activator activity / Long-term potentiation / Regulation of MECP2 expression and activity / Calcineurin activates NFAT / regulation of ryanodine-sensitive calcium-release channel activity / DARPP-32 events / voltage-gated potassium channel activity / action potential / catalytic complex / Smooth Muscle Contraction / regulation of cardiac muscle contraction / detection of calcium ion / RHO GTPases activate IQGAPs / calcium channel inhibitor activity / regulation of cardiac muscle contraction by regulation of the release of sequestered calcium ion / presynaptic cytosol / cellular response to interferon-beta / Protein methylation / Activation of AMPK downstream of NMDARs / positive regulation of protein autophosphorylation / regulation of release of sequestered calcium ion into cytosol by sarcoplasmic reticulum / Ion homeostasis / eNOS activation / regulation of calcium-mediated signaling / titin binding / Tetrahydrobiopterin (BH4) synthesis, recycling, salvage and regulation / voltage-gated potassium channel complex / sperm midpiece / calcium channel complex / potassium ion transmembrane transport / substantia nigra development / positive regulation of peptidyl-threonine phosphorylation / calyx of Held / FCERI mediated Ca+2 mobilization / adenylate cyclase activator activity / Ras activation upon Ca2+ influx through NMDA receptor / FCGR3A-mediated IL10 synthesis / regulation of heart rate / sarcomere / protein serine/threonine kinase activator activity / Antigen activates B Cell Receptor (BCR) leading to generation of second messengers / regulation of cytokinesis / VEGFR2 mediated cell proliferation / VEGFR2 mediated vascular permeability / Translocation of SLC2A4 (GLUT4) to the plasma membrane / positive regulation of protein serine/threonine kinase activity / spindle microtubule / positive regulation of receptor signaling pathway via JAK-STAT / RAF activation / Transcriptional activation of mitochondrial biogenesis / Stimuli-sensing channels / cellular response to type II interferon / spindle pole / response to calcium ion / calcium-dependent protein binding / RAS processing / Signaling by RAF1 mutants / Signaling by moderate kinase activity BRAF mutants / Paradoxical activation of RAF signaling by kinase inactive BRAF / Signaling downstream of RAS mutants / G2/M transition of mitotic cell cycle 類似検索 - 分子機能 | |||||||||
| 生物種 |  Homo sapiens (ヒト) Homo sapiens (ヒト) | |||||||||
| 手法 | 単粒子再構成法 / クライオ電子顕微鏡法 / 解像度: 3.4 Å | |||||||||
 データ登録者 データ登録者 | Ma D / Guo J | |||||||||
| 資金援助 |  中国, 2件 中国, 2件
| |||||||||
 引用 引用 |  ジャーナル: Nat Chem Biol / 年: 2024 ジャーナル: Nat Chem Biol / 年: 2024タイトル: A small-molecule activation mechanism that directly opens the KCNQ2 channel. 著者: Shaoying Zhang / Demin Ma / Kun Wang / Ya Li / Zhenni Yang / Xiaoxiao Li / Junnan Li / Jiangnan He / Lianghe Mei / Yangliang Ye / Zongsheng Chen / Juwen Shen / Panpan Hou / Jiangtao Guo / ...著者: Shaoying Zhang / Demin Ma / Kun Wang / Ya Li / Zhenni Yang / Xiaoxiao Li / Junnan Li / Jiangnan He / Lianghe Mei / Yangliang Ye / Zongsheng Chen / Juwen Shen / Panpan Hou / Jiangtao Guo / Qiansen Zhang / Huaiyu Yang /  要旨: Pharmacological activation of voltage-gated ion channels by ligands serves as the basis for therapy and mainly involves a classic gating mechanism that augments the native voltage-dependent open ...Pharmacological activation of voltage-gated ion channels by ligands serves as the basis for therapy and mainly involves a classic gating mechanism that augments the native voltage-dependent open probability. Through structure-based virtual screening, we identified a new scaffold compound, Ebio1, serving as a potent and subtype-selective activator for the voltage-gated potassium channel KCNQ2 and featuring a new activation mechanism. Single-channel patch-clamp, cryogenic-electron microscopy and molecular dynamic simulations, along with chemical derivatives, reveal that Ebio1 engages the KCNQ2 activation by generating an extended channel gate with a larger conductance at the saturating voltage (+50 mV). This mechanism is different from the previously observed activation mechanism of ligands on voltage-gated ion channels. Ebio1 caused S6 helices from residues S303 and F305 to perform a twist-to-open movement, which was sufficient to open the KCNQ2 gate. Overall, our findings provide mechanistic insights into the activation of KCNQ2 channel by Ebio1 and lend support for KCNQ-related drug development. | |||||||||
| 履歴 |
|
- 構造の表示
構造の表示
| 添付画像 |
|---|
- ダウンロードとリンク
ダウンロードとリンク
-EMDBアーカイブ
| マップデータ |  emd_35487.map.gz emd_35487.map.gz | 47.8 MB |  EMDBマップデータ形式 EMDBマップデータ形式 | |
|---|---|---|---|---|
| ヘッダ (付随情報) |  emd-35487-v30.xml emd-35487-v30.xml emd-35487.xml emd-35487.xml | 15.8 KB 15.8 KB | 表示 表示 |  EMDBヘッダ EMDBヘッダ |
| 画像 |  emd_35487.png emd_35487.png | 58 KB | ||
| Filedesc metadata |  emd-35487.cif.gz emd-35487.cif.gz | 5.9 KB | ||
| その他 |  emd_35487_half_map_1.map.gz emd_35487_half_map_1.map.gz emd_35487_half_map_2.map.gz emd_35487_half_map_2.map.gz | 37.1 MB 37.1 MB | ||
| アーカイブディレクトリ |  http://ftp.pdbj.org/pub/emdb/structures/EMD-35487 http://ftp.pdbj.org/pub/emdb/structures/EMD-35487 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-35487 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-35487 | HTTPS FTP |
-検証レポート
| 文書・要旨 |  emd_35487_validation.pdf.gz emd_35487_validation.pdf.gz | 964.8 KB | 表示 |  EMDB検証レポート EMDB検証レポート |
|---|---|---|---|---|
| 文書・詳細版 |  emd_35487_full_validation.pdf.gz emd_35487_full_validation.pdf.gz | 964.4 KB | 表示 | |
| XML形式データ |  emd_35487_validation.xml.gz emd_35487_validation.xml.gz | 11.6 KB | 表示 | |
| CIF形式データ |  emd_35487_validation.cif.gz emd_35487_validation.cif.gz | 13.5 KB | 表示 | |
| アーカイブディレクトリ |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-35487 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-35487 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-35487 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-35487 | HTTPS FTP |
-関連構造データ
| 関連構造データ |  8ijkMC  8x43C M: このマップから作成された原子モデル C: 同じ文献を引用 ( |
|---|---|
| 類似構造データ | 類似検索 - 機能・相同性  F&H 検索 F&H 検索 |
- リンク
リンク
| EMDBのページ |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| 「今月の分子」の関連する項目 |
- マップ
マップ
| ファイル |  ダウンロード / ファイル: emd_35487.map.gz / 形式: CCP4 / 大きさ: 52.7 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) ダウンロード / ファイル: emd_35487.map.gz / 形式: CCP4 / 大きさ: 52.7 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 投影像・断面図 | 画像のコントロール
画像は Spider により作成 | ||||||||||||||||||||||||||||||||||||
| ボクセルのサイズ | X=Y=Z: 0.93 Å | ||||||||||||||||||||||||||||||||||||
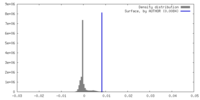
| 密度 |
| ||||||||||||||||||||||||||||||||||||
| 対称性 | 空間群: 1 | ||||||||||||||||||||||||||||||||||||
| 詳細 | EMDB XML:
|
-添付データ
-ハーフマップ: #1
| ファイル | emd_35487_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 投影像・断面図 |
| ||||||||||||
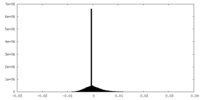
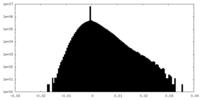
| 密度ヒストグラム |
-ハーフマップ: #2
| ファイル | emd_35487_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 投影像・断面図 |
| ||||||||||||
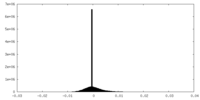
| 密度ヒストグラム |
- 試料の構成要素
試料の構成要素
-全体 : human KCNQ2-CaM-Ebio1 complex in the presence of PIP2
| 全体 | 名称: human KCNQ2-CaM-Ebio1 complex in the presence of PIP2 |
|---|---|
| 要素 |
|
-超分子 #1: human KCNQ2-CaM-Ebio1 complex in the presence of PIP2
| 超分子 | 名称: human KCNQ2-CaM-Ebio1 complex in the presence of PIP2 タイプ: complex / ID: 1 / 親要素: 0 / 含まれる分子: #1-#2 |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
-分子 #1: Potassium voltage-gated channel subfamily KQT member 2
| 分子 | 名称: Potassium voltage-gated channel subfamily KQT member 2 タイプ: protein_or_peptide / ID: 1 / コピー数: 4 / 光学異性体: LEVO |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
| 分子量 | 理論値: 73.627812 KDa |
| 組換発現 | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
| 配列 | 文字列: MAGKPPKRNA FYRKLQNFLY NVLERPRGWA FIYHAYVFLL VFSCLVLSVF STIKEYEKSS EGALYILEIV TIVVFGVEYF VRIWAAGCC CRYRGWRGRL KFARKPFCVI DIMVLIASIA VLAAGSQGNV FATSALRSLR FLQILRMIRM DRRGGTWKLL G SVVYAHSK ...文字列: MAGKPPKRNA FYRKLQNFLY NVLERPRGWA FIYHAYVFLL VFSCLVLSVF STIKEYEKSS EGALYILEIV TIVVFGVEYF VRIWAAGCC CRYRGWRGRL KFARKPFCVI DIMVLIASIA VLAAGSQGNV FATSALRSLR FLQILRMIRM DRRGGTWKLL G SVVYAHSK ELVTAWYIGF LCLILASFLV YLAEKGENDH FDTYADALWW GLITLTTIGY GDKYPQTWNG RLLAATFTLI GV SFFALPA GILGSGFALK VQEQHRQKHF EKRRNPAAGL IQSAWRFYAT NLSRTDLHST WQYYERTVTV PMYSSQTQTY GAS RLIPPL NQLELLRNLK SKSGLAFRKD PPPEPSPSKG SPCRGPLCGC CPGRSSQKVS LKDRVFSSPR GVAAKGKGSP QAQT VRRSP SADQSLEDSP SKVPKSWSFG DRSRARQAFR IKGAASRQNS EEASLPGEDI VDDKSCPCEF VTEDLTPGLK VSIRA VCVM RFLVSKRKFK ESLRPYDVMD VIEQYSAGHL DMLSRIKSLQ SRVDQIVGRG PAITDKDRTK GPAEAELPED PSMMGR LGK VEKQVLSMEK KLDFLVNIYM QRMGIPPTET EAYFGAKEPE PAPPYHSPED SREHVDRHGC IVKIVRSSSS TGQKNFS VE GGSSGGWSHP QFEK UniProtKB: Potassium voltage-gated channel subfamily KQT member 2 |
-分子 #2: Calmodulin-1
| 分子 | 名称: Calmodulin-1 / タイプ: protein_or_peptide / ID: 2 / コピー数: 4 / 光学異性体: LEVO |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
| 分子量 | 理論値: 16.852545 KDa |
| 組換発現 | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
| 配列 | 文字列: MADQLTEEQI AEFKEAFSLF DKDGDGTITT KELGTVMRSL GQNPTEAELQ DMINEVDADG NGTIDFPEFL TMMARKMKDT DSEEEIREA FRVFDKDGNG YISAAELRHV MTNLGEKLTD EEVDEMIREA DIDGDGQVNY EEFVQMMTAK UniProtKB: Calmodulin-1 |
-分子 #3: N-(1,2-dihydroacenaphthylen-5-yl)-4-fluoranyl-benzamide
| 分子 | 名称: N-(1,2-dihydroacenaphthylen-5-yl)-4-fluoranyl-benzamide タイプ: ligand / ID: 3 / コピー数: 4 / 式: 7PN |
|---|---|
| 分子量 | 理論値: 291.319 Da |
| Chemical component information |  ChemComp-7PN: |
-実験情報
-構造解析
| 手法 | クライオ電子顕微鏡法 |
|---|---|
 解析 解析 | 単粒子再構成法 |
| 試料の集合状態 | particle |
- 試料調製
試料調製
| 緩衝液 | pH: 8 |
|---|---|
| 凍結 | 凍結剤: ETHANE |
- 電子顕微鏡法
電子顕微鏡法
| 顕微鏡 | FEI TITAN KRIOS |
|---|---|
| 撮影 | フィルム・検出器のモデル: FEI FALCON IV (4k x 4k) 平均電子線量: 52.0 e/Å2 |
| 電子線 | 加速電圧: 300 kV / 電子線源:  FIELD EMISSION GUN FIELD EMISSION GUN |
| 電子光学系 | 照射モード: FLOOD BEAM / 撮影モード: BRIGHT FIELD / 最大 デフォーカス(公称値): 1.5 µm / 最小 デフォーカス(公称値): 0.8 µm |
| 実験機器 |  モデル: Titan Krios / 画像提供: FEI Company |
- 画像解析
画像解析
| 初期モデル | モデルのタイプ: EMDB MAP EMDB ID: |
|---|---|
| 最終 再構成 | 解像度のタイプ: BY AUTHOR / 解像度: 3.4 Å / 解像度の算出法: FSC 0.143 CUT-OFF / 使用した粒子像数: 28232 |
| 初期 角度割当 | タイプ: MAXIMUM LIKELIHOOD |
| 最終 角度割当 | タイプ: MAXIMUM LIKELIHOOD |
 ムービー
ムービー コントローラー
コントローラー


























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)