+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| タイトル | Cryo-EM structure of human HCAR2-Gi complex with acipimox | |||||||||
 マップデータ マップデータ | ||||||||||
 試料 試料 |
| |||||||||
 キーワード キーワード | complex / MEMBRANE PROTEIN | |||||||||
| 機能・相同性 |  機能・相同性情報 機能・相同性情報nicotinic acid receptor activity / Hydroxycarboxylic acid-binding receptors / neutrophil apoptotic process / positive regulation of neutrophil apoptotic process / Class A/1 (Rhodopsin-like receptors) / positive regulation of adiponectin secretion / T cell migration / negative regulation of lipid catabolic process / Adenylate cyclase inhibitory pathway / positive regulation of protein localization to cell cortex ...nicotinic acid receptor activity / Hydroxycarboxylic acid-binding receptors / neutrophil apoptotic process / positive regulation of neutrophil apoptotic process / Class A/1 (Rhodopsin-like receptors) / positive regulation of adiponectin secretion / T cell migration / negative regulation of lipid catabolic process / Adenylate cyclase inhibitory pathway / positive regulation of protein localization to cell cortex / regulation of cAMP-mediated signaling / D2 dopamine receptor binding / G protein-coupled serotonin receptor binding / regulation of mitotic spindle organization / cellular response to forskolin / adenylate cyclase-inhibiting G protein-coupled receptor signaling pathway / Regulation of insulin secretion / G protein-coupled receptor binding / G-protein beta/gamma-subunit complex binding / Olfactory Signaling Pathway / Activation of the phototransduction cascade / adenylate cyclase-modulating G protein-coupled receptor signaling pathway / G beta:gamma signalling through PLC beta / Presynaptic function of Kainate receptors / Thromboxane signalling through TP receptor / G protein-coupled acetylcholine receptor signaling pathway / G-protein activation / Activation of G protein gated Potassium channels / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / Prostacyclin signalling through prostacyclin receptor / response to peptide hormone / Glucagon signaling in metabolic regulation / G beta:gamma signalling through CDC42 / G beta:gamma signalling through BTK / ADP signalling through P2Y purinoceptor 12 / Sensory perception of sweet, bitter, and umami (glutamate) taste / Synthesis, secretion, and inactivation of Glucagon-like Peptide-1 (GLP-1) / photoreceptor disc membrane / Glucagon-type ligand receptors / Adrenaline,noradrenaline inhibits insulin secretion / Vasopressin regulates renal water homeostasis via Aquaporins / G alpha (z) signalling events / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / cellular response to catecholamine stimulus / ADORA2B mediated anti-inflammatory cytokines production / sensory perception of taste / ADP signalling through P2Y purinoceptor 1 / G beta:gamma signalling through PI3Kgamma / adenylate cyclase-activating dopamine receptor signaling pathway / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / GPER1 signaling / GDP binding / cellular response to prostaglandin E stimulus / Inactivation, recovery and regulation of the phototransduction cascade / G-protein beta-subunit binding / heterotrimeric G-protein complex / G alpha (12/13) signalling events / extracellular vesicle / cell junction / signaling receptor complex adaptor activity / Thrombin signalling through proteinase activated receptors (PARs) / GTPase binding / retina development in camera-type eye / Ca2+ pathway / phospholipase C-activating G protein-coupled receptor signaling pathway / cell cortex / midbody / G alpha (i) signalling events / fibroblast proliferation / 加水分解酵素; 酸無水物に作用; GTPに作用・細胞または細胞小器官の運動に関与 / G alpha (s) signalling events / G alpha (q) signalling events / Ras protein signal transduction / cell population proliferation / Extra-nuclear estrogen signaling / G protein-coupled receptor signaling pathway / cell division / lysosomal membrane / GTPase activity / centrosome / synapse / protein-containing complex binding / nucleolus / GTP binding / magnesium ion binding / signal transduction / extracellular exosome / nucleoplasm / membrane / plasma membrane / cytosol / cytoplasm 類似検索 - 分子機能 | |||||||||
| 生物種 |  Homo sapiens (ヒト) Homo sapiens (ヒト) | |||||||||
| 手法 | 単粒子再構成法 / クライオ電子顕微鏡法 / 解像度: 3.23 Å | |||||||||
 データ登録者 データ登録者 | Pan X / Fang Y | |||||||||
| 資金援助 |  中国, 1件 中国, 1件
| |||||||||
 引用 引用 |  ジャーナル: Cell Discov / 年: 2023 ジャーナル: Cell Discov / 年: 2023タイトル: Structural insights into ligand recognition and selectivity of the human hydroxycarboxylic acid receptor HCAR2. 著者: Xin Pan / Fang Ye / Peiruo Ning / Zhiyi Zhang / Xinyu Li / Binghao Zhang / Qian Wang / Geng Chen / Wei Gao / Chen Qiu / Zhangsong Wu / Jiancheng Li / Lizhe Zhu / Jiang Xia / Kaizheng Gong / Yang Du /  要旨: Hydroxycarboxylic acid receptor 2 (HCAR2) belongs to the family of class A G protein-coupled receptors with key roles in regulating lipolysis and free fatty acid formation in humans. It is deeply ...Hydroxycarboxylic acid receptor 2 (HCAR2) belongs to the family of class A G protein-coupled receptors with key roles in regulating lipolysis and free fatty acid formation in humans. It is deeply involved in many pathophysiological processes and serves as an attractive target for the treatment of cardiovascular, neoplastic, autoimmune, neurodegenerative, inflammatory, and metabolic diseases. Here, we report four cryo-EM structures of human HCAR2-Gi1 complexes with or without agonists, including the drugs niacin (2.69 Å) and acipimox (3.23 Å), the highly subtype-specific agonist MK-6892 (3.25 Å), and apo form (3.28 Å). Combined with molecular dynamics simulation and functional analysis, we have revealed the recognition mechanism of HCAR2 for different agonists and summarized the general pharmacophore features of HCAR2 agonists, which are based on three key residues R111, S179, and Y284. Notably, the MK-6892-HCAR2 structure shows an extended binding pocket relative to other agonist-bound HCAR2 complexes. In addition, the key residues that determine the ligand selectivity between the HCAR2 and HCAR3 are also illuminated. Our findings provide structural insights into the ligand recognition, selectivity, activation, and G protein coupling mechanism of HCAR2, which shed light on the design of new HCAR2-targeting drugs for greater efficacy, higher selectivity, and fewer or no side effects. | |||||||||
| 履歴 |
|
- 構造の表示
構造の表示
| 添付画像 |
|---|
- ダウンロードとリンク
ダウンロードとリンク
-EMDBアーカイブ
| マップデータ |  emd_35484.map.gz emd_35484.map.gz | 64.3 MB |  EMDBマップデータ形式 EMDBマップデータ形式 | |
|---|---|---|---|---|
| ヘッダ (付随情報) |  emd-35484-v30.xml emd-35484-v30.xml emd-35484.xml emd-35484.xml | 18.9 KB 18.9 KB | 表示 表示 |  EMDBヘッダ EMDBヘッダ |
| 画像 |  emd_35484.png emd_35484.png | 109.6 KB | ||
| Filedesc metadata |  emd-35484.cif.gz emd-35484.cif.gz | 6.4 KB | ||
| その他 |  emd_35484_half_map_1.map.gz emd_35484_half_map_1.map.gz emd_35484_half_map_2.map.gz emd_35484_half_map_2.map.gz | 117.9 MB 118 MB | ||
| アーカイブディレクトリ |  http://ftp.pdbj.org/pub/emdb/structures/EMD-35484 http://ftp.pdbj.org/pub/emdb/structures/EMD-35484 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-35484 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-35484 | HTTPS FTP |
-検証レポート
| 文書・要旨 |  emd_35484_validation.pdf.gz emd_35484_validation.pdf.gz | 752 KB | 表示 |  EMDB検証レポート EMDB検証レポート |
|---|---|---|---|---|
| 文書・詳細版 |  emd_35484_full_validation.pdf.gz emd_35484_full_validation.pdf.gz | 751.5 KB | 表示 | |
| XML形式データ |  emd_35484_validation.xml.gz emd_35484_validation.xml.gz | 13.9 KB | 表示 | |
| CIF形式データ |  emd_35484_validation.cif.gz emd_35484_validation.cif.gz | 16.4 KB | 表示 | |
| アーカイブディレクトリ |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-35484 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-35484 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-35484 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-35484 | HTTPS FTP |
-関連構造データ
- リンク
リンク
| EMDBのページ |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| 「今月の分子」の関連する項目 |
- マップ
マップ
| ファイル |  ダウンロード / ファイル: emd_35484.map.gz / 形式: CCP4 / 大きさ: 125 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) ダウンロード / ファイル: emd_35484.map.gz / 形式: CCP4 / 大きさ: 125 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 投影像・断面図 | 画像のコントロール
画像は Spider により作成 | ||||||||||||||||||||||||||||||||||||
| ボクセルのサイズ | X=Y=Z: 0.85 Å | ||||||||||||||||||||||||||||||||||||
| 密度 |
| ||||||||||||||||||||||||||||||||||||
| 対称性 | 空間群: 1 | ||||||||||||||||||||||||||||||||||||
| 詳細 | EMDB XML:
|
-添付データ
-ハーフマップ: #2
| ファイル | emd_35484_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 投影像・断面図 |
| ||||||||||||
| 密度ヒストグラム |
-ハーフマップ: #1
| ファイル | emd_35484_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 投影像・断面図 |
| ||||||||||||
| 密度ヒストグラム |
- 試料の構成要素
試料の構成要素
-全体 : Cryo-EM structure of human HCAR2-Gi complex with acipimox
| 全体 | 名称: Cryo-EM structure of human HCAR2-Gi complex with acipimox |
|---|---|
| 要素 |
|
-超分子 #1: Cryo-EM structure of human HCAR2-Gi complex with acipimox
| 超分子 | 名称: Cryo-EM structure of human HCAR2-Gi complex with acipimox タイプ: complex / ID: 1 / 親要素: 0 / 含まれる分子: #1-#5 |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
-分子 #1: Hydroxycarboxylic acid receptor 2
| 分子 | 名称: Hydroxycarboxylic acid receptor 2 / タイプ: protein_or_peptide / ID: 1 / コピー数: 1 / 光学異性体: LEVO |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
| 分子量 | 理論値: 34.251973 KDa |
| 組換発現 | 生物種:  |
| 配列 | 文字列: DHFLEIDKKN CCVFRDDFIV KVLPPVLGLE FIFGLLGNGL ALWIFCFHLK SWKSSRIFLF NLAVADFLLI ICLPFLMDNY VRRWDWKFG DIPCRLMLFM LAMNRQGSII FLTVVAVDRY FRVVHPHHAL NKISNRTAAI ISCLLWGITI GLTVHLLKKK M PIQNGGAN ...文字列: DHFLEIDKKN CCVFRDDFIV KVLPPVLGLE FIFGLLGNGL ALWIFCFHLK SWKSSRIFLF NLAVADFLLI ICLPFLMDNY VRRWDWKFG DIPCRLMLFM LAMNRQGSII FLTVVAVDRY FRVVHPHHAL NKISNRTAAI ISCLLWGITI GLTVHLLKKK M PIQNGGAN LCSSFSICHT FQWHEAMFLL EFFLPLGIIL FCSARIIWSL RQRQMDRHAK IKRAITFIMV VAIVFVICFL PS VVVRIRI FWLLHTSGTQ NCEVYRSVDL AFFITLSFTY MNSMLDPVVY YFSSPSF UniProtKB: Hydroxycarboxylic acid receptor 2 |
-分子 #2: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1
| 分子 | 名称: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 タイプ: protein_or_peptide / ID: 2 / コピー数: 1 / 光学異性体: LEVO |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
| 分子量 | 理論値: 37.069543 KDa |
| 組換発現 | 生物種:  |
| 配列 | 文字列: LDQLRQEAEQ LKNQIRDARK ACADATLSQI TNNIDPVGRI QMRTRRTLRG HLAKIYAMHW GTDSRLLVSA SQDGKLIIWD SYTTNKVHA IPLRSSWVMT CAYAPSGNYV ACGGLDNICS IYNLKTREGN VRVSRELAGH TGYLSCCRFL DDNQIVTSSG D TTCALWDI ...文字列: LDQLRQEAEQ LKNQIRDARK ACADATLSQI TNNIDPVGRI QMRTRRTLRG HLAKIYAMHW GTDSRLLVSA SQDGKLIIWD SYTTNKVHA IPLRSSWVMT CAYAPSGNYV ACGGLDNICS IYNLKTREGN VRVSRELAGH TGYLSCCRFL DDNQIVTSSG D TTCALWDI ETGQQTTTFT GHTGDVMSLS LAPDTRLFVS GACDASAKLW DVREGMCRQT FTGHESDINA ICFFPNGNAF AT GSDDATC RLFDLRADQE LMTYSHDNII CGITSVSFSK SGRLLLAGYD DFNCNVWDAL KADRAGVLAG HDNRVSCLGV TDD GMAVAT GSWDSFLKIW N UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 |
-分子 #3: Guanine nucleotide-binding protein G(i) subunit alpha-1
| 分子 | 名称: Guanine nucleotide-binding protein G(i) subunit alpha-1 タイプ: protein_or_peptide / ID: 3 / コピー数: 1 / 光学異性体: LEVO |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
| 分子量 | 理論値: 40.153672 KDa |
| 組換発現 | 生物種:  |
| 配列 | 文字列: TLSAEDKAAV ERSKMIDRNL REDGEKAARE VKLLLLGAGE SGKSTIVKQM KIIHEAGYSE EECKQYKAVV YSNTIQSIIA IIRAMGRLK IDFGDSARAD DARQLFVLAG AAEEGFMTAE LAGVIKRLWK DSGVQACFNR SREYQLNDSA AYYLNDLDRI A QPNYIPTQ ...文字列: TLSAEDKAAV ERSKMIDRNL REDGEKAARE VKLLLLGAGE SGKSTIVKQM KIIHEAGYSE EECKQYKAVV YSNTIQSIIA IIRAMGRLK IDFGDSARAD DARQLFVLAG AAEEGFMTAE LAGVIKRLWK DSGVQACFNR SREYQLNDSA AYYLNDLDRI A QPNYIPTQ QDVLRTRVKT TGIVETHFTF KDLHFKMFDV GAQRSERKKW IHCFEGVTAI IFCVALSDYD LVLAEDEEMN RM HESMKLF DSICNNKWFT DTSIILFLNK KDLFEEKIKK SPLTICYPEY AGSNTYEEAA AYIQCQFEDL NKRKDTKEIY THF TCSTDT KNVQFVFDAV TDVIIKNNLK DCGLF UniProtKB: Guanine nucleotide-binding protein G(i) subunit alpha-1 |
-分子 #4: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2
| 分子 | 名称: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 タイプ: protein_or_peptide / ID: 4 / コピー数: 1 / 光学異性体: LEVO |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
| 分子量 | 理論値: 6.218162 KDa |
| 組換発現 | 生物種:  |
| 配列 | 文字列: SIAQARKLVE QLKMEANIDR IKVSKAAADL MAYCEAHAKE DPLLTPVPAS ENPFRE UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 |
-分子 #5: scFv16
| 分子 | 名称: scFv16 / タイプ: protein_or_peptide / ID: 5 / コピー数: 1 / 光学異性体: LEVO |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
| 分子量 | 理論値: 26.424385 KDa |
| 組換発現 | 生物種:  |
| 配列 | 文字列: DVQLVESGGG LVQPGGSRKL SCSASGFAFS SFGMHWVRQA PEKGLEWVAY ISSGSGTIYY ADTVKGRFTI SRDDPKNTLF LQMTSLRSE DTAMYYCVRS IYYYGSSPFD FWGQGTTLTV SSGGGGSGGG GSGGGGSSDI VMTQATSSVP VTPGESVSIS C RSSKSLLH ...文字列: DVQLVESGGG LVQPGGSRKL SCSASGFAFS SFGMHWVRQA PEKGLEWVAY ISSGSGTIYY ADTVKGRFTI SRDDPKNTLF LQMTSLRSE DTAMYYCVRS IYYYGSSPFD FWGQGTTLTV SSGGGGSGGG GSGGGGSSDI VMTQATSSVP VTPGESVSIS C RSSKSLLH SNGNTYLYWF LQRPGQSPQL LIYRMSNLAS GVPDRFSGSG SGTAFTLTIS RLEAEDVGVY YCMQHLEYPL TF GAGTKLE L |
-分子 #6: 5-methyl-4-oxidanyl-pyrazin-4-ium-2-carboxylic acid
| 分子 | 名称: 5-methyl-4-oxidanyl-pyrazin-4-ium-2-carboxylic acid / タイプ: ligand / ID: 6 / コピー数: 1 / 式: OJX |
|---|---|
| 分子量 | 理論値: 155.131 Da |
| Chemical component information | 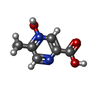 ChemComp-OJX: |
-実験情報
-構造解析
| 手法 | クライオ電子顕微鏡法 |
|---|---|
 解析 解析 | 単粒子再構成法 |
| 試料の集合状態 | particle |
- 試料調製
試料調製
| 緩衝液 | pH: 7.2 |
|---|---|
| 凍結 | 凍結剤: NITROGEN |
- 電子顕微鏡法
電子顕微鏡法
| 顕微鏡 | FEI TITAN KRIOS |
|---|---|
| 撮影 | フィルム・検出器のモデル: GATAN K3 (6k x 4k) / 平均電子線量: 56.0 e/Å2 |
| 電子線 | 加速電圧: 300 kV / 電子線源:  FIELD EMISSION GUN FIELD EMISSION GUN |
| 電子光学系 | 照射モード: SPOT SCAN / 撮影モード: BRIGHT FIELD 最大 デフォーカス(公称値): 1.4000000000000001 µm 最小 デフォーカス(公称値): 1.0 µm |
| 実験機器 |  モデル: Titan Krios / 画像提供: FEI Company |
- 画像解析
画像解析
| 初期モデル | モデルのタイプ: NONE |
|---|---|
| 最終 再構成 | 解像度のタイプ: BY AUTHOR / 解像度: 3.23 Å / 解像度の算出法: FSC 0.143 CUT-OFF / 使用した粒子像数: 221940 |
| 初期 角度割当 | タイプ: NOT APPLICABLE |
| 最終 角度割当 | タイプ: NOT APPLICABLE |
 ムービー
ムービー コントローラー
コントローラー







































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)




































