+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
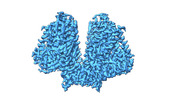
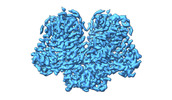
| Title | AtOSCA1.1 extended state | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | channel / MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationregulation of calcium ion import / calcium-activated cation channel activity / cellular hyperosmotic response / response to osmotic stress / monoatomic cation channel activity / protein tetramerization / plasma membrane / cytosol Similarity search - Function | |||||||||
| Biological species |   | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.5 Å | |||||||||
 Authors Authors | Zhang MF | |||||||||
| Funding support | 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2023 Journal: Nat Commun / Year: 2023Title: A mechanical-coupling mechanism in OSCA/TMEM63 channel mechanosensitivity. Authors: Mingfeng Zhang / Yuanyue Shan / Charles D Cox / Duanqing Pei /   Abstract: Mechanosensitive (MS) ion channels are a ubiquitous type of molecular force sensor sensing forces from the surrounding bilayer. The profound structural diversity in these channels suggests that the ...Mechanosensitive (MS) ion channels are a ubiquitous type of molecular force sensor sensing forces from the surrounding bilayer. The profound structural diversity in these channels suggests that the molecular mechanisms of force sensing follow unique structural blueprints. Here we determine the structures of plant and mammalian OSCA/TMEM63 proteins, allowing us to identify essential elements for mechanotransduction and propose roles for putative bound lipids in OSCA/TMEM63 mechanosensation. Briefly, the central cavity created by the dimer interface couples each subunit and modulates dimeric OSCA/TMEM63 channel mechanosensitivity through the modulating lipids while the cytosolic side of the pore is gated by a plug lipid that prevents the ion permeation. Our results suggest that the gating mechanism of OSCA/TMEM63 channels may combine structural aspects of the 'lipid-gated' mechanism of MscS and TRAAK channels and the calcium-induced gating mechanism of the TMEM16 family, which may provide insights into the structural rearrangements of TMEM16/TMC superfamilies. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_34209.map.gz emd_34209.map.gz | 229.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-34209-v30.xml emd-34209-v30.xml emd-34209.xml emd-34209.xml | 13.5 KB 13.5 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_34209.png emd_34209.png | 76.3 KB | ||
| Filedesc metadata |  emd-34209.cif.gz emd-34209.cif.gz | 5.6 KB | ||
| Others |  emd_34209_half_map_1.map.gz emd_34209_half_map_1.map.gz emd_34209_half_map_2.map.gz emd_34209_half_map_2.map.gz | 226.2 MB 226.2 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-34209 http://ftp.pdbj.org/pub/emdb/structures/EMD-34209 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-34209 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-34209 | HTTPS FTP |
-Validation report
| Summary document |  emd_34209_validation.pdf.gz emd_34209_validation.pdf.gz | 1.1 MB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_34209_full_validation.pdf.gz emd_34209_full_validation.pdf.gz | 1.1 MB | Display | |
| Data in XML |  emd_34209_validation.xml.gz emd_34209_validation.xml.gz | 16 KB | Display | |
| Data in CIF |  emd_34209_validation.cif.gz emd_34209_validation.cif.gz | 18.7 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-34209 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-34209 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-34209 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-34209 | HTTPS FTP |
-Related structure data
| Related structure data |  8grnMC  8groC  8grsC  8gsoC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_34209.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_34209.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Voxel size | X=Y=Z: 0.849 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_34209_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
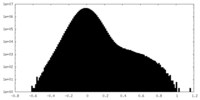
| Projections & Slices |
| ||||||||||||
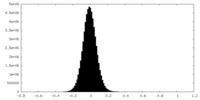
| Density Histograms |
-Half map: #1
| File | emd_34209_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
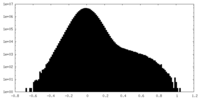
| Projections & Slices |
| ||||||||||||
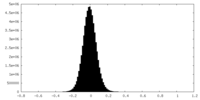
| Density Histograms |
- Sample components
Sample components
-Entire : AtOSCA1.1 extended state
| Entire | Name: AtOSCA1.1 extended state |
|---|---|
| Components |
|
-Supramolecule #1: AtOSCA1.1 extended state
| Supramolecule | Name: AtOSCA1.1 extended state / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Protein OSCA1
| Macromolecule | Name: Protein OSCA1 / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 87.697008 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MATLKDIGVS AGINILTAFI FFIIFAFLRL QPFNDRVYFS KWYLRGLRSS PASGGGFAGR FVNLELRSYL KFLHWMPEAL KMPERELID HAGLDSVVYL RIYWLGLKIF APIAMLAWAV LVPVNWTNNE LELAKHFKNV TSSDIDKLTI SNIPEGSNRF W AHIIMAYA ...String: MATLKDIGVS AGINILTAFI FFIIFAFLRL QPFNDRVYFS KWYLRGLRSS PASGGGFAGR FVNLELRSYL KFLHWMPEAL KMPERELID HAGLDSVVYL RIYWLGLKIF APIAMLAWAV LVPVNWTNNE LELAKHFKNV TSSDIDKLTI SNIPEGSNRF W AHIIMAYA FTIWTCYMLM KEYETVANMR LQFLASEGRR PDQFTVLVRN VPPDPDETVS ELVEHFFLVN HPDNYLTHQV VC NANKLAD LVSKKTKLQN WLDYYQLKYT RNNSQIRPIT KLGCLGLCGQ KVDAIEHYIA EVDKTSKEIA EERENVVNDQ KSV MPASFV SFKTRWAAAV CAQTTQTRNP TEWLTEWAAE PRDIYWPNLA IPYVSLTVRR LVMNVAFFFL TFFFIIPIAF VQSL ATIEG IEKVAPFLKV IIEKDFIKSL IQGLLAGIAL KLFLIFLPAI LMTMSKFEGF TSVSFLERRS ASRYYIFNLV NVFLG SVIA GAAFEQLNSF LNQSPNQIPK TIGMAIPMKA TFFITYIMVD GWAGVAGEIL MLKPLIIYHL KNAFLVKTEK DREEAM NPG SIGFNTGEPQ IQLYFLLGLV YAPVTPMLLP FILVFFALAY VVYRHQIINV YNQEYESAAA FWPDVHGRVI TALIISQ LL LMGLLGTKHA ASAAPFLIAL PVITIGFHRF CKGRFEPAFV RYPLQEAMMK DTLERAREPN LNLKGYLQDA YIHPVFKG G DNDDDGDMIG KLENEVIIVP TKRQSRRNTP APSRISGESS PSLAVINGKE V UniProtKB: Protein OSCA1 |
-Macromolecule #2: [1-MYRISTOYL-GLYCEROL-3-YL]PHOSPHONYLCHOLINE
| Macromolecule | Name: [1-MYRISTOYL-GLYCEROL-3-YL]PHOSPHONYLCHOLINE / type: ligand / ID: 2 / Number of copies: 2 / Formula: LPC |
|---|---|
| Molecular weight | Theoretical: 468.585 Da |
| Chemical component information |  ChemComp-LPC: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: SPOT SCAN / Imaging mode: BRIGHT FIELD / Nominal defocus max: 3.0 µm / Nominal defocus min: 2.0 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: NONE |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 2.5 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 838000 |
| Initial angle assignment | Type: NOT APPLICABLE |
| Final angle assignment | Type: NOT APPLICABLE |
 Movie
Movie Controller
Controller





 Z
Z Y
Y X
X