[English] 日本語
 Yorodumi
Yorodumi- EMDB-34157: structure of human connexin 40.1 pentameric hemichannel by cryoEM -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | structure of human connexin 40.1 pentameric hemichannel by cryoEM | ||||||||||||
 Map data Map data | |||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | connexin hemichannel / MEMBRANE PROTEIN | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationregulation of satellite cell activation involved in skeletal muscle regeneration / connexin complex / Gap junction assembly / gap junction channel activity / cell-cell signaling Similarity search - Function | ||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.5 Å | ||||||||||||
 Authors Authors | Zhang H / Wang DP | ||||||||||||
| Funding support |  China, 3 items China, 3 items
| ||||||||||||
 Citation Citation |  Journal: To Be Published Journal: To Be PublishedTitle: structure of human connexin 40.1 intercellular gap junction channel by cryoEM Authors: Zhang H / Wang DP | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_34157.map.gz emd_34157.map.gz | 59.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-34157-v30.xml emd-34157-v30.xml emd-34157.xml emd-34157.xml | 12.9 KB 12.9 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_34157.png emd_34157.png | 50.3 KB | ||
| Filedesc metadata |  emd-34157.cif.gz emd-34157.cif.gz | 4.9 KB | ||
| Others |  emd_34157_half_map_1.map.gz emd_34157_half_map_1.map.gz emd_34157_half_map_2.map.gz emd_34157_half_map_2.map.gz | 59.3 MB 59.3 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-34157 http://ftp.pdbj.org/pub/emdb/structures/EMD-34157 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-34157 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-34157 | HTTPS FTP |
-Validation report
| Summary document |  emd_34157_validation.pdf.gz emd_34157_validation.pdf.gz | 817 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_34157_full_validation.pdf.gz emd_34157_full_validation.pdf.gz | 816.6 KB | Display | |
| Data in XML |  emd_34157_validation.xml.gz emd_34157_validation.xml.gz | 12.5 KB | Display | |
| Data in CIF |  emd_34157_validation.cif.gz emd_34157_validation.cif.gz | 14.6 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-34157 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-34157 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-34157 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-34157 | HTTPS FTP |
-Related structure data
| Related structure data |  8gn8MC  8gn7C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_34157.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_34157.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.92 Å | ||||||||||||||||||||||||||||||||||||
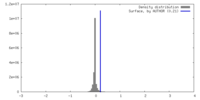
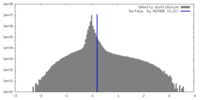
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #1
| File | emd_34157_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
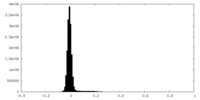
| Density Histograms |
-Half map: #2
| File | emd_34157_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
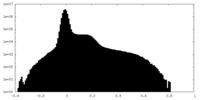
| Density Histograms |
- Sample components
Sample components
-Entire : structure of human connexin 40.1 pentameric hemichannel
| Entire | Name: structure of human connexin 40.1 pentameric hemichannel |
|---|---|
| Components |
|
-Supramolecule #1: structure of human connexin 40.1 pentameric hemichannel
| Supramolecule | Name: structure of human connexin 40.1 pentameric hemichannel type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 130 KDa |
-Macromolecule #1: Gap junction delta-4 protein
| Macromolecule | Name: Gap junction delta-4 protein / type: protein_or_peptide / ID: 1 / Number of copies: 5 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 26.030973 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MEGVDLLGFL IITLNCNVTM VGKLWFVLTM LLRMLVIVLA GRPVYQDEQE RFVCNTLQPG CANVCYDVFS PVSHLRFWLI QGVCVLLPS AVFSVYVLHR GATLAALGPR RCPDPREPAS GQRRCPRPFG ERGGLQVPDF SAGYIIHLLL RTLLEAAFGA L HYFLFGFL ...String: MEGVDLLGFL IITLNCNVTM VGKLWFVLTM LLRMLVIVLA GRPVYQDEQE RFVCNTLQPG CANVCYDVFS PVSHLRFWLI QGVCVLLPS AVFSVYVLHR GATLAALGPR RCPDPREPAS GQRRCPRPFG ERGGLQVPDF SAGYIIHLLL RTLLEAAFGA L HYFLFGFL APKKFPCTRP PCTGVVDCYV SRPTEKSLLM LFLWAVSALS FLLGLADLVC SLRRRMRRRP GPPTS UniProtKB: Gap junction delta-4 protein |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 4 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.5 µm / Nominal defocus min: 1.0 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: OTHER |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.5 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 62658 |
| Initial angle assignment | Type: ANGULAR RECONSTITUTION |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller




 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)