+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | SIDT2-pH5.5 plus miRNA | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | membrane protein | |||||||||
| Function / homology |  Function and homology information Function and homology informationnucleic acid transmembrane transporter activity / AP-1 adaptor complex binding / RNA transmembrane transporter activity / AP-2 adaptor complex binding / RNA transport / type B pancreatic cell development / regulation of insulin secretion involved in cellular response to glucose stimulus / type B pancreatic cell proliferation / RNA catabolic process / response to glucose ...nucleic acid transmembrane transporter activity / AP-1 adaptor complex binding / RNA transmembrane transporter activity / AP-2 adaptor complex binding / RNA transport / type B pancreatic cell development / regulation of insulin secretion involved in cellular response to glucose stimulus / type B pancreatic cell proliferation / RNA catabolic process / response to glucose / cell morphogenesis / double-stranded RNA binding / glucose homeostasis / lysosome / lysosomal membrane / DNA binding / plasma membrane Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
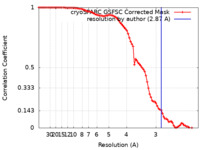
| Method | single particle reconstruction / cryo EM / Resolution: 2.87 Å | |||||||||
 Authors Authors | Gong DS | |||||||||
| Funding support | 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2023 Journal: Nat Commun / Year: 2023Title: Structural insight into the human SID1 transmembrane family member 2 reveals its lipid hydrolytic activity. Authors: Dandan Qian / Ye Cong / Runhao Wang / Quan Chen / Chuangye Yan / Deshun Gong /  Abstract: The systemic RNAi-defective (SID) transmembrane family member 2 (SIDT2) is a putative nucleic acid channel or transporter that plays essential roles in nucleic acid transport and lipid metabolism. ...The systemic RNAi-defective (SID) transmembrane family member 2 (SIDT2) is a putative nucleic acid channel or transporter that plays essential roles in nucleic acid transport and lipid metabolism. Here, we report the cryo-electron microscopy (EM) structures of human SIDT2, which forms a tightly packed dimer with extensive interactions mediated by two previously uncharacterized extracellular/luminal β-strand-rich domains and the unique transmembrane domain (TMD). The TMD of each SIDT2 protomer contains eleven transmembrane helices (TMs), and no discernible nucleic acid conduction pathway has been identified within the TMD, suggesting that it may act as a transporter. Intriguingly, TM3-6 and TM9-11 form a large cavity with a putative catalytic zinc atom coordinated by three conserved histidine residues and one aspartate residue lying approximately 6 Å from the extracellular/luminal surface of the membrane. Notably, SIDT2 can hydrolyze C18 ceramide into sphingosine and fatty acid with a slow rate. The information presented advances the understanding of the structure-function relationships in the SID1 family proteins. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_33637.map.gz emd_33637.map.gz | 59.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-33637-v30.xml emd-33637-v30.xml emd-33637.xml emd-33637.xml | 14 KB 14 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_33637_fsc.xml emd_33637_fsc.xml | 8.4 KB | Display |  FSC data file FSC data file |
| Images |  emd_33637.png emd_33637.png | 123 KB | ||
| Filedesc metadata |  emd-33637.cif.gz emd-33637.cif.gz | 5.6 KB | ||
| Others |  emd_33637_half_map_1.map.gz emd_33637_half_map_1.map.gz emd_33637_half_map_2.map.gz emd_33637_half_map_2.map.gz | 59.1 MB 59.1 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-33637 http://ftp.pdbj.org/pub/emdb/structures/EMD-33637 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-33637 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-33637 | HTTPS FTP |
-Validation report
| Summary document |  emd_33637_validation.pdf.gz emd_33637_validation.pdf.gz | 904.2 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_33637_full_validation.pdf.gz emd_33637_full_validation.pdf.gz | 903.8 KB | Display | |
| Data in XML |  emd_33637_validation.xml.gz emd_33637_validation.xml.gz | 16.5 KB | Display | |
| Data in CIF |  emd_33637_validation.cif.gz emd_33637_validation.cif.gz | 21.4 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-33637 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-33637 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-33637 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-33637 | HTTPS FTP |
-Related structure data
| Related structure data |  7y68MC  7y63C  7y69C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_33637.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_33637.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.0825 Å | ||||||||||||||||||||||||||||||||||||
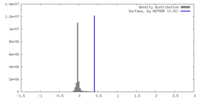
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_33637_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
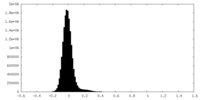
| Density Histograms |
-Half map: #1
| File | emd_33637_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
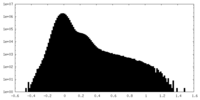
| Density Histograms |
- Sample components
Sample components
-Entire : dimer of SIDT2
| Entire | Name: dimer of SIDT2 |
|---|---|
| Components |
|
-Supramolecule #1: dimer of SIDT2
| Supramolecule | Name: dimer of SIDT2 / type: organelle_or_cellular_component / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: SID1 transmembrane family member 2
| Macromolecule | Name: SID1 transmembrane family member 2 / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 94.539938 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MFALGLPFLV LLVASVESHL GVLGPKNVSQ KDAEFERTYV DEVNSELVNI YTFNHTVTRN RTEGVRVSVN VLNKQKGAPL LFVVRQKEA VVSFQVPLIL RGMFQRKYLY QKVERTLCQP PTKNESEIQF FYVDVSTLSP VNTTYQLRVS RMDDFVLRTG E QFSFNTTA ...String: MFALGLPFLV LLVASVESHL GVLGPKNVSQ KDAEFERTYV DEVNSELVNI YTFNHTVTRN RTEGVRVSVN VLNKQKGAPL LFVVRQKEA VVSFQVPLIL RGMFQRKYLY QKVERTLCQP PTKNESEIQF FYVDVSTLSP VNTTYQLRVS RMDDFVLRTG E QFSFNTTA AQPQYFKYEF PEGVDSVIVK VTSNKAFPCS VISIQDVLCP VYDLDNNVAF IGMYQTMTKK AAITVQRKDF PS NSFYVVV VVKTEDQACG GSLPFYPFAE DEPVDQGHRQ KTLSVLVSQA VTSEAYVSGM LFCLGIFLSF YLLTVLLACW ENW RQKKKT LLVAIDRACP ESGHPRVLAD SFPGSSPYEG YNYGSFENVS GSTDGLVDSA GTGDLSYGYQ GRSFEPVGTR PRVD SMSSV EEDDYDTLTD IDSDKNVIRT KQYLYVADLA RKDKRVLRKK YQIYFWNIAT IAVFYALPVV QLVITYQTVV NVTGN QDIC YYNFLCAHPL GNLSAFNNIL SNLGYILLGL LFLLIILQRE INHNRALLRN DLCALECGIP KHFGLFYAMG TALMME GLL SACYHVCPNY TNFQFDTSFM YMIAGLCMLK LYQKRHPDIN ASAYSAYACL AIVIFFSVLG VVFGKGNTAF WIVFSII HI IATLLLSTQL YYMGRWKLDS GIFRRILHVL YTDCIRQCSG PLYVDRMVLL VMGNVINWSL AAYGLIMRPN DFASYLLA I GICNLLLYFA FYIIMKLRSG ERIKLIPLLC IVCTSVVWGF ALFFFFQGLS TWQKTPAESR EHNRDCILLD FFDDHDIWH FLSSIAMFGS FLVLLTLDDD LDTVQRDKIY VF UniProtKB: SID1 transmembrane family member 2 |
-Macromolecule #3: ZINC ION
| Macromolecule | Name: ZINC ION / type: ligand / ID: 3 / Number of copies: 2 / Formula: ZN |
|---|---|
| Molecular weight | Theoretical: 65.409 Da |
-Macromolecule #4: 2-acetamido-2-deoxy-beta-D-glucopyranose
| Macromolecule | Name: 2-acetamido-2-deoxy-beta-D-glucopyranose / type: ligand / ID: 4 / Number of copies: 8 / Formula: NAG |
|---|---|
| Molecular weight | Theoretical: 221.208 Da |
| Chemical component information |  ChemComp-NAG: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 1.8 µm / Nominal defocus min: 1.3 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller






 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)