[English] 日本語
 Yorodumi
Yorodumi- EMDB-33572: Complex of integrin alphaV/beta8 and L-TGF-beta1 at a ratio of 1:2 -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Complex of integrin alphaV/beta8 and L-TGF-beta1 at a ratio of 1:2 | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
| Function / homology |  Function and homology information Function and homology informationganglioside metabolic process / adaptive immune response based on somatic recombination of immune receptors built from immunoglobulin superfamily domains / regulation of interleukin-23 production / branch elongation involved in mammary gland duct branching / positive regulation of primary miRNA processing / regulation of branching involved in mammary gland duct morphogenesis / positive regulation of microglia differentiation / Influenza Virus Induced Apoptosis / negative regulation of skeletal muscle tissue development / frontal suture morphogenesis ...ganglioside metabolic process / adaptive immune response based on somatic recombination of immune receptors built from immunoglobulin superfamily domains / regulation of interleukin-23 production / branch elongation involved in mammary gland duct branching / positive regulation of primary miRNA processing / regulation of branching involved in mammary gland duct morphogenesis / positive regulation of microglia differentiation / Influenza Virus Induced Apoptosis / negative regulation of skeletal muscle tissue development / frontal suture morphogenesis / regulation of enamel mineralization / regulation of cartilage development / TGFBR2 MSI Frameshift Mutants in Cancer / regulatory T cell differentiation / regulation of blood vessel remodeling / tolerance induction to self antigen / regulation of striated muscle tissue development / regulation of protein import into nucleus / negative regulation of natural killer cell mediated cytotoxicity directed against tumor cell target / embryonic liver development / columnar/cuboidal epithelial cell maturation / negative regulation of hyaluronan biosynthetic process / type III transforming growth factor beta receptor binding / positive regulation of cardiac muscle cell differentiation / myofibroblast differentiation / Langerhans cell differentiation / positive regulation of odontogenesis / positive regulation of receptor signaling pathway via STAT / positive regulation of exit from mitosis / extracellular matrix assembly / connective tissue replacement involved in inflammatory response wound healing / integrin alphav-beta8 complex / integrin alphav-beta6 complex / hard palate development / transforming growth factor beta production / odontoblast differentiation / negative regulation of entry of bacterium into host cell / integrin alphav-beta5 complex / TGFBR2 Kinase Domain Mutants in Cancer / opsonin binding / negative regulation of macrophage cytokine production / positive regulation of smooth muscle cell differentiation / positive regulation of mesenchymal stem cell proliferation / positive regulation of isotype switching to IgA isotypes / integrin alphav-beta1 complex / SMAD2/3 Phosphorylation Motif Mutants in Cancer / TGFBR1 KD Mutants in Cancer / mammary gland branching involved in thelarche / TGFBR3 regulates TGF-beta signaling / retina vasculature development in camera-type eye / Cross-presentation of particulate exogenous antigens (phagosomes) / membrane protein intracellular domain proteolysis / extracellular matrix protein binding / response to laminar fluid shear stress / heart valve morphogenesis / positive regulation of vasculature development / bronchiole development / hyaluronan catabolic process / Laminin interactions / ATP biosynthetic process / positive regulation of extracellular matrix assembly / receptor catabolic process / lens fiber cell differentiation / placenta blood vessel development / positive regulation of branching involved in ureteric bud morphogenesis / negative regulation of extracellular matrix disassembly / negative regulation of lipoprotein metabolic process / integrin alphav-beta3 complex / negative regulation of low-density lipoprotein receptor activity / TGFBR1 LBD Mutants in Cancer / type II transforming growth factor beta receptor binding / oligodendrocyte development / alphav-beta3 integrin-PKCalpha complex / germ cell migration / negative regulation of biomineral tissue development / positive regulation of mononuclear cell migration / response to salt / alphav-beta3 integrin-HMGB1 complex / type I transforming growth factor beta receptor binding / phospholipid homeostasis / regulation of phagocytosis / positive regulation of chemotaxis / endoderm development / negative regulation of lipid transport / negative regulation of myoblast differentiation / positive regulation of vascular permeability / digestive tract development / positive regulation of endothelial cell apoptotic process / cell-cell junction organization / Elastic fibre formation / response to vitamin D / positive regulation of regulatory T cell differentiation / alphav-beta3 integrin-IGF-1-IGF1R complex / response to cholesterol / transforming growth factor beta binding / entry into host cell by a symbiont-containing vacuole / negative regulation of interleukin-17 production / surfactant homeostasis / deubiquitinase activator activity / positive regulation of small GTPase mediated signal transduction Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.24 Å | |||||||||
 Authors Authors | Duan Z / Zhang Z | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2022 Journal: Nat Commun / Year: 2022Title: Specificity of TGF-β1 signal designated by LRRC33 and integrin αβ. Authors: Zelin Duan / Xuezhen Lin / Lixia Wang / Qiuxin Zhen / Yuefeng Jiang / Chuxin Chen / Jing Yang / Chia-Hsueh Lee / Yan Qin / Ying Li / Bo Zhao / Jianchuan Wang / Zhe Zhang /   Abstract: Myeloid lineage cells present the latent form of transforming growth factor-β1 (L-TGF-β1) to the membrane using an anchor protein LRRC33. Integrin αβ activates extracellular L-TGF-β1 to trigger ...Myeloid lineage cells present the latent form of transforming growth factor-β1 (L-TGF-β1) to the membrane using an anchor protein LRRC33. Integrin αβ activates extracellular L-TGF-β1 to trigger the downstream signaling functions. However, the mechanism designating the specificity of TGF-β1 presentation and activation remains incompletely understood. Here, we report cryo-EM structures of human L-TGF-β1/LRRC33 and integrin αβ/L-TGF-β1 complexes. Combined with biochemical and cell-based analyses, we demonstrate that LRRC33 only presents L-TGF-β1 but not the -β2 or -β3 isoforms due to difference of key residues on the growth factor domains. Moreover, we reveal a 2:2 binding mode of integrin αβ and L-TGF-β1, which shows higher avidity and more efficient L-TGF-β1 activation than previously reported 1:2 binding mode. We also uncover that the disulfide-linked loop of the integrin subunit β determines its exquisite affinity to L-TGF-β1. Together, our findings provide important insights into the specificity of TGF-β1 signaling achieved by LRRC33 and integrin αβ. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_33572.map.gz emd_33572.map.gz | 204.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-33572-v30.xml emd-33572-v30.xml emd-33572.xml emd-33572.xml | 18.5 KB 18.5 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_33572.png emd_33572.png | 107.2 KB | ||
| Others |  emd_33572_half_map_1.map.gz emd_33572_half_map_1.map.gz emd_33572_half_map_2.map.gz emd_33572_half_map_2.map.gz | 200.2 MB 200.2 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-33572 http://ftp.pdbj.org/pub/emdb/structures/EMD-33572 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-33572 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-33572 | HTTPS FTP |
-Validation report
| Summary document |  emd_33572_validation.pdf.gz emd_33572_validation.pdf.gz | 791.6 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_33572_full_validation.pdf.gz emd_33572_full_validation.pdf.gz | 791.2 KB | Display | |
| Data in XML |  emd_33572_validation.xml.gz emd_33572_validation.xml.gz | 15.6 KB | Display | |
| Data in CIF |  emd_33572_validation.cif.gz emd_33572_validation.cif.gz | 18.5 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-33572 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-33572 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-33572 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-33572 | HTTPS FTP |
-Related structure data
| Related structure data |  7y1tMC  7y1rC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_33572.map.gz / Format: CCP4 / Size: 216 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_33572.map.gz / Format: CCP4 / Size: 216 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.055 Å | ||||||||||||||||||||||||||||||||||||
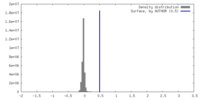
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #1
| File | emd_33572_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
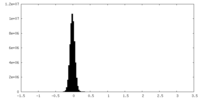
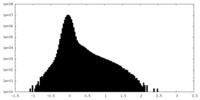
| Density Histograms |
-Half map: #2
| File | emd_33572_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
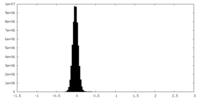
| Density Histograms |
- Sample components
Sample components
-Entire : Complex of integrin alphaV/beta8 and L-TGF-beta1 at a ratio of 1:2
| Entire | Name: Complex of integrin alphaV/beta8 and L-TGF-beta1 at a ratio of 1:2 |
|---|---|
| Components |
|
-Supramolecule #1: Complex of integrin alphaV/beta8 and L-TGF-beta1 at a ratio of 1:2
| Supramolecule | Name: Complex of integrin alphaV/beta8 and L-TGF-beta1 at a ratio of 1:2 type: complex / Chimera: Yes / ID: 1 / Parent: 0 / Macromolecule list: #1-#3 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Recombinant expression | Organism:  Homo sapiens (human) / Recombinant cell: HEK293 Homo sapiens (human) / Recombinant cell: HEK293 |
| Molecular weight | Theoretical: 210 KDa |
-Macromolecule #1: Integrin alpha-V
| Macromolecule | Name: Integrin alpha-V / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 69.812711 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MAFPPRRRLR LGPRGLPLLL SGLLLPLCRA FNLDVDSPAE YSGPEGSYFG FAVDFFVPSA SSRMFLLVGA PKANTTQPGI VEGGQVLKC DWSSTRRCQP IEFDATGNRD YAKDDPLEFK SHQWFGASVR SKQDKILACA PLYHWRTEMK QEREPVGTCF L QDGTKTVE ...String: MAFPPRRRLR LGPRGLPLLL SGLLLPLCRA FNLDVDSPAE YSGPEGSYFG FAVDFFVPSA SSRMFLLVGA PKANTTQPGI VEGGQVLKC DWSSTRRCQP IEFDATGNRD YAKDDPLEFK SHQWFGASVR SKQDKILACA PLYHWRTEMK QEREPVGTCF L QDGTKTVE YAPCRSQDID ADGQGFCQGG FSIDFTKADR VLLGGPGSFY WQGQLISDQV AEIVSKYDPN VYSIKYNNQL AT RTAQAIF DDSYLGYSVA VGDFNGDGID DFVSGVPRAA RTLGMVYIYD GKNMSSLYNF TGEQMAAYFG FSVAATDING DDY ADVFIG APLFMDRGSD GKLQEVGQVS VSLQRASGDF QTTKLNGFEV FARFGSAIAP LGDLDQDGFN DIAIAAPYGG EDKK GIVYI FNGRSTGLNA VPSQILEGQW AARSGCPPSF GYSMKGATDI DKNGYPDLIV GAFGVDRAIL YRARPVITVN AGLEV YPSI LNQDNKTCSL PGTALKVSCF NVRFCLKADG KGVLPRKLNF QVELLLDKLK QKGAIRRALF LYSRSPSHSK NMTISR GGL MQCEELIAYL RDESEFRDKL TPITIFMEYR LDYRTAADTT GLQPILNQFT PANISRQAHI LLDTGGLEVL FQ |
-Macromolecule #2: Integrin beta-8
| Macromolecule | Name: Integrin beta-8 / type: protein_or_peptide / ID: 2 Details: The first 21 residues (MDMRVPAQLLGLLLLWFSGVL) are signal peptides. Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 53.017355 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MDMRVPAQLL GLLLLWFSGV LEDNRCASSN AASCARCLAL GPECGWCVQE DFISGGSRSE RCDIVSNLIS KGCSVDSIEY PSVHVIIPT ENEINTQVTP GEVSIQLRPG AEANFMLKVH PLKKYPVDLY YLVDVSASMH NNIEKLNSVG NDLSRKMAFF S RDFRLGFG ...String: MDMRVPAQLL GLLLLWFSGV LEDNRCASSN AASCARCLAL GPECGWCVQE DFISGGSRSE RCDIVSNLIS KGCSVDSIEY PSVHVIIPT ENEINTQVTP GEVSIQLRPG AEANFMLKVH PLKKYPVDLY YLVDVSASMH NNIEKLNSVG NDLSRKMAFF S RDFRLGFG SYVDKTVSPY ISIHPERIHN QCSDYNLDCM PPHGYIHVLS LTENITEFEK AVHRQKISGN IDTPEGGFDA ML QAAVCES HIGWRKEAKR LLLVMTDQTS HLALDSKLAG IVCPNDGNCH LKNNVYVKST TMEHPSLGQL SEKLIDNNIN VIF AVQGKQ FHWYKDLLPL LPGTIAGEIE SKAANLNNLV VEAYQKLISE VKVQVENQVQ GIYFNITAIC PDGSRKPGME GCRN VTSND EVLFNVTVTM KKCDVTGGKN YAIIKPIGFN ETAKIHIHRN CSCQCEDNRG PKGKCVDETF LDSKCFQCDE NK |
-Macromolecule #3: Transforming growth factor beta-1 proprotein
| Macromolecule | Name: Transforming growth factor beta-1 proprotein / type: protein_or_peptide / ID: 3 Details: The first 16 residues (MPLLLLLPLLWAGALA) are signal peptides. Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 43.010402 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MPLLLLLPLL WAGALALSTC KTIDMELVKR KRIEAIRGQI LSKLRLASPP SQGEVPPGPL PEAVLALYNS TRDRVAGESA EPEPEPEAD YYAKEVTRVL MVETHNEIYD KFKQSTHSIY MFFNTSELRE AVPEPVLLSR AELRLLRLKL KVEQHVELYQ K YSNNSWRY ...String: MPLLLLLPLL WAGALALSTC KTIDMELVKR KRIEAIRGQI LSKLRLASPP SQGEVPPGPL PEAVLALYNS TRDRVAGESA EPEPEPEAD YYAKEVTRVL MVETHNEIYD KFKQSTHSIY MFFNTSELRE AVPEPVLLSR AELRLLRLKL KVEQHVELYQ K YSNNSWRY LSNRLLAPSD SPEWLSFDVT GVVRQWLSRG GEIEGFRLSA HCSCDSRDNT LQVDINGFTT GRRGDLATIH GM NRPFLLL MATPLERAQH LQSSRHRRAL DTNYCFSSTE KNCCVRQLYI DFRKDLGWKW IHEPKGYHAN FCLGPCPYIW SLD TQYSKV LALYNQHNPG ASAAPCCVPQ ALEPLPIVYY VGRKPKVEQL SNMIVRSCKC S |
-Macromolecule #7: 2-acetamido-2-deoxy-beta-D-glucopyranose
| Macromolecule | Name: 2-acetamido-2-deoxy-beta-D-glucopyranose / type: ligand / ID: 7 / Number of copies: 5 / Formula: NAG |
|---|---|
| Molecular weight | Theoretical: 221.208 Da |
| Chemical component information |  ChemComp-NAG: |
-Macromolecule #8: CALCIUM ION
| Macromolecule | Name: CALCIUM ION / type: ligand / ID: 8 / Number of copies: 5 / Formula: CA |
|---|---|
| Molecular weight | Theoretical: 40.078 Da |
-Macromolecule #9: MANGANESE (II) ION
| Macromolecule | Name: MANGANESE (II) ION / type: ligand / ID: 9 / Number of copies: 1 / Formula: MN |
|---|---|
| Molecular weight | Theoretical: 54.938 Da |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 3 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: SUPER-RESOLUTION / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 1.2 µm / Nominal defocus min: 0.8 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.24 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 305011 |
|---|---|
| Initial angle assignment | Type: NOT APPLICABLE |
| Final angle assignment | Type: ANGULAR RECONSTITUTION |
-Atomic model buiding 1
| Refinement | Protocol: OTHER |
|---|---|
| Output model |  PDB-7y1t: |
 Movie
Movie Controller
Controller






















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)