[English] 日本語
 Yorodumi
Yorodumi- EMDB-33031: Cryo-EM structure of Streptomyces coelicolor transcription initia... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of Streptomyces coelicolor transcription initial complex with two Zur dimers | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | DNA BINDING PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationregulation of secondary metabolite biosynthetic process / bacterial-type RNA polymerase holo enzyme binding / DNA-binding transcription repressor activity / sigma factor activity / DNA-directed RNA polymerase complex / DNA-templated transcription initiation / protein-DNA complex / ribonucleoside binding / DNA-directed 5'-3' RNA polymerase activity / DNA-directed RNA polymerase ...regulation of secondary metabolite biosynthetic process / bacterial-type RNA polymerase holo enzyme binding / DNA-binding transcription repressor activity / sigma factor activity / DNA-directed RNA polymerase complex / DNA-templated transcription initiation / protein-DNA complex / ribonucleoside binding / DNA-directed 5'-3' RNA polymerase activity / DNA-directed RNA polymerase / transcription cis-regulatory region binding / protein dimerization activity / DNA-binding transcription factor activity / negative regulation of DNA-templated transcription / DNA-templated transcription / magnesium ion binding / DNA binding / zinc ion binding / cytosol / cytoplasm Similarity search - Function | |||||||||
| Biological species |  Streptomyces coelicolor A3(2) (bacteria) Streptomyces coelicolor A3(2) (bacteria) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.7 Å | |||||||||
 Authors Authors | Yang X / Zheng J | |||||||||
| Funding support | 1 items
| |||||||||
 Citation Citation |  Journal: Nucleic Acids Res / Year: 2022 Journal: Nucleic Acids Res / Year: 2022Title: Structural basis of Streptomyces transcription activation by zinc uptake regulator. Authors: Xu Yang / Yiqun Wang / Guiyang Liu / Zixin Deng / Shuangjun Lin / Jianting Zheng /  Abstract: Streptomyces coelicolor (Sc) is a model organism of actinobacteria to study morphological differentiation and production of bioactive metabolites. Sc zinc uptake regulator (Zur) affects both ...Streptomyces coelicolor (Sc) is a model organism of actinobacteria to study morphological differentiation and production of bioactive metabolites. Sc zinc uptake regulator (Zur) affects both processes by controlling zinc homeostasis. It activates transcription by binding to palindromic Zur-box sequences upstream of -35 elements. Here we deciphered the molecular mechanism by which ScZur interacts with promoter DNA and Sc RNA polymerase (RNAP) by cryo-EM structures and biochemical assays. The ScZur-DNA structures reveal a sequential and cooperative binding of three ScZur dimers surrounding a Zur-box spaced 8 nt upstream from a -35 element. The ScRNAPσHrdB-Zur-DNA structures define protein-protein and protein-DNA interactions involved in the principal housekeeping σHrdB-dependent transcription initiation from a noncanonical promoter with a -10 element lacking the critical adenine residue at position -11 and a TTGCCC -35 element deviating from the canonical TTGACA motif. ScZur interacts with the C-terminal domain of ScRNAP α subunit (αCTD) in a complex structure trapped in an active conformation. Key ScZur-αCTD interfacial residues accounting for ScZur-dependent transcription activation were confirmed by mutational studies. Together, our structural and biochemical results provide a comprehensive model for transcription activation of Zur family regulators. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_33031.map.gz emd_33031.map.gz | 6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-33031-v30.xml emd-33031-v30.xml emd-33031.xml emd-33031.xml | 27.6 KB 27.6 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_33031_fsc.xml emd_33031_fsc.xml | 12.8 KB | Display |  FSC data file FSC data file |
| Images |  emd_33031.png emd_33031.png | 18.7 KB | ||
| Masks |  emd_33031_msk_1.map emd_33031_msk_1.map | 216 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-33031.cif.gz emd-33031.cif.gz | 8.6 KB | ||
| Others |  emd_33031_half_map_1.map.gz emd_33031_half_map_1.map.gz emd_33031_half_map_2.map.gz emd_33031_half_map_2.map.gz | 200.2 MB 200.2 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-33031 http://ftp.pdbj.org/pub/emdb/structures/EMD-33031 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-33031 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-33031 | HTTPS FTP |
-Validation report
| Summary document |  emd_33031_validation.pdf.gz emd_33031_validation.pdf.gz | 796.6 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_33031_full_validation.pdf.gz emd_33031_full_validation.pdf.gz | 796.2 KB | Display | |
| Data in XML |  emd_33031_validation.xml.gz emd_33031_validation.xml.gz | 21.6 KB | Display | |
| Data in CIF |  emd_33031_validation.cif.gz emd_33031_validation.cif.gz | 28.2 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-33031 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-33031 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-33031 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-33031 | HTTPS FTP |
-Related structure data
| Related structure data |  7x74MC  7vo0C  7vo9C  7vpdC  7vpzC  7x75C  7x76C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_33031.map.gz / Format: CCP4 / Size: 216 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_33031.map.gz / Format: CCP4 / Size: 216 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.1 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_33031_msk_1.map emd_33031_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
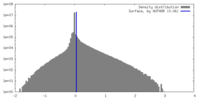
| Density Histograms |
-Half map: #2
| File | emd_33031_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_33031_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
+Entire : Cryo-EM structure of Streptomyces coelicolor transcription initia...
+Supramolecule #1: Cryo-EM structure of Streptomyces coelicolor transcription initia...
+Macromolecule #1: DNA-directed RNA polymerase subunit alpha
+Macromolecule #2: DNA-directed RNA polymerase subunit beta
+Macromolecule #3: DNA-directed RNA polymerase subunit beta'
+Macromolecule #4: DNA-directed RNA polymerase subunit omega
+Macromolecule #5: RNA polymerase principal sigma factor HrdB
+Macromolecule #6: Putative metal uptake regulation protein
+Macromolecule #7: DNA (84-MER)
+Macromolecule #8: DNA (84-MER)
+Macromolecule #9: RNA
+Macromolecule #10: MAGNESIUM ION
+Macromolecule #11: ZINC ION
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.8 mg/mL | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 8 Component:
| ||||||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 289 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 QUANTUM (4k x 4k) / Average electron dose: 40.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.0 µm / Nominal defocus min: 1.0 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller
















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)