+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
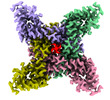
| Title | Ion channel | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | ion channel / TRANSPORT PROTEIN | |||||||||
| Biological species |  Emiliania huxleyi (eukaryote) Emiliania huxleyi (eukaryote) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.83 Å | |||||||||
 Authors Authors | Jiang D / Zhang J | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2022 Journal: Nat Commun / Year: 2022Title: N-type fast inactivation of a eukaryotic voltage-gated sodium channel. Authors: Jiangtao Zhang / Yiqiang Shi / Junping Fan / Huiwen Chen / Zhanyi Xia / Bo Huang / Juquan Jiang / Jianke Gong / Zhuo Huang / Daohua Jiang /  Abstract: Voltage-gated sodium (Na) channels initiate action potentials. Fast inactivation of Na channels, mediated by an Ile-Phe-Met motif, is crucial for preventing hyperexcitability and regulating firing ...Voltage-gated sodium (Na) channels initiate action potentials. Fast inactivation of Na channels, mediated by an Ile-Phe-Met motif, is crucial for preventing hyperexcitability and regulating firing frequency. Here we present cryo-electron microscopy structure of NaEh from the coccolithophore Emiliania huxleyi, which reveals an unexpected molecular gating mechanism for Na channel fast inactivation independent of the Ile-Phe-Met motif. An N-terminal helix of NaEh plugs into the open activation gate and blocks it. The binding pose of the helix is stabilized by multiple electrostatic interactions. Deletion of the helix or mutations blocking the electrostatic interactions completely abolished the fast inactivation. These strong interactions enable rapid inactivation, but also delay recovery from fast inactivation, which is ~160-fold slower than human Na channels. Together, our results provide mechanistic insights into fast inactivation of NaEh that fundamentally differs from the conventional local allosteric inhibition, revealing both surprising structural diversity and functional conservation of ion channel inactivation. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_33016.map.gz emd_33016.map.gz | 59.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-33016-v30.xml emd-33016-v30.xml emd-33016.xml emd-33016.xml | 12.5 KB 12.5 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_33016.png emd_33016.png | 151.9 KB | ||
| Filedesc metadata |  emd-33016.cif.gz emd-33016.cif.gz | 5.8 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-33016 http://ftp.pdbj.org/pub/emdb/structures/EMD-33016 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-33016 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-33016 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_33016.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_33016.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.04 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
- Sample components
Sample components
-Entire : ion channel complex
| Entire | Name: ion channel complex |
|---|---|
| Components |
|
-Supramolecule #1: ion channel complex
| Supramolecule | Name: ion channel complex / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Emiliania huxleyi (eukaryote) Emiliania huxleyi (eukaryote) |
-Macromolecule #1: ion channel,GFP-TwinStrep
| Macromolecule | Name: ion channel,GFP-TwinStrep / type: protein_or_peptide / ID: 1 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Emiliania huxleyi (eukaryote) Emiliania huxleyi (eukaryote) |
| Molecular weight | Theoretical: 91.684469 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MIAAIHNARR KKREAAAAHK AQHRTAENSM DSLEDSTHET DAGERAQAGS TKLAWTDVVA PPPRKVVFWL PHQRKVFDFY ASQGVQYFT AFLIVSNFIF NCAEKEWDPY TDQLYQGLWR WGEFAFNTMF LIELLINFYG IAFCFWRYNW AWNTFDLVVV A IGTLTMAE ...String: MIAAIHNARR KKREAAAAHK AQHRTAENSM DSLEDSTHET DAGERAQAGS TKLAWTDVVA PPPRKVVFWL PHQRKVFDFY ASQGVQYFT AFLIVSNFIF NCAEKEWDPY TDQLYQGLWR WGEFAFNTMF LIELLINFYG IAFCFWRYNW AWNTFDLVVV A IGTLTMAE AIGGNFMPPS MALIRNLRAF RIFRLFKRIK SLNKIIVSLG KAIPGVANAF VIMVIIMCIY AILGVEFYHM TG SDGTYVT YNDNVKRGLC TGDEVELGQC SLNQTVSSET ARGYTYGEEY YGTFFRALYT LFQVLTGESW SEAVARPAVF ESH YDSFGP VLFYVSFIII CQIVLINVVV AVLLDKMVEE DDSEDPEKQT VAEKLSEMLS QEHAQLREIF RTWDEDNSGT ISIK EWRKA VKSMGYRGPI DVLDQIFASM DKDHSGELDY AEIDRMLSPT AARERRSSTH ANPKRSVKEE VVAMRAEFTD HVARL ETQI AALVLELQLQ RKPCGAEAPA PAHSRLAHDS DGAPTEPPPP AAPDHHHLED DEDTTQRVAA ALEVLFQGPS KGEELF TGV VPILVELDGD VNGHKFSVRG EGEGDATNGK LTLKFICTTG KLPVPWPTLV TTLTYGVQCF SRYPDHMKRH DFFKSAM PE GYVQERTISF KDDGTYKTRA EVKFEGDTLV NRIELKGIDF KEDGNILGHK LEYNFNSHNV YITADKQKNG IKANFKIR H NVEDGSVQLA DHYQQNTPIG DGPVLLPDNH YLSTQSVLSK DPNEKRDHMV LLEFVTAAGI THGMDEWSHP QFEKGGGSG GGSGGSAWSH PQFEK |
-Macromolecule #2: ion channel
| Macromolecule | Name: ion channel / type: protein_or_peptide / ID: 2 / Details: N-terminal helix of either of the four protomers / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Emiliania huxleyi (eukaryote) Emiliania huxleyi (eukaryote) |
| Molecular weight | Theoretical: 1.913298 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MIAAIHNARR KKREAAA |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.5 µm / Nominal defocus min: 1.2 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller




 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)




















