+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of dodecamer P97 | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | AAA ATPase / CYTOSOLIC PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationcytoplasmic ubiquitin ligase complex / flavin adenine dinucleotide catabolic process / VCP-NSFL1C complex / endoplasmic reticulum stress-induced pre-emptive quality control / endosome to lysosome transport via multivesicular body sorting pathway / BAT3 complex binding / cellular response to arsenite ion / protein-DNA covalent cross-linking repair / Derlin-1 retrotranslocation complex / positive regulation of protein K63-linked deubiquitination ...cytoplasmic ubiquitin ligase complex / flavin adenine dinucleotide catabolic process / VCP-NSFL1C complex / endoplasmic reticulum stress-induced pre-emptive quality control / endosome to lysosome transport via multivesicular body sorting pathway / BAT3 complex binding / cellular response to arsenite ion / protein-DNA covalent cross-linking repair / Derlin-1 retrotranslocation complex / positive regulation of protein K63-linked deubiquitination / cytoplasm protein quality control / positive regulation of oxidative phosphorylation / aggresome assembly / deubiquitinase activator activity / mitotic spindle disassembly / ubiquitin-modified protein reader activity / regulation of protein localization to chromatin / VCP-NPL4-UFD1 AAA ATPase complex / ciliary transition zone / cellular response to misfolded protein / positive regulation of mitochondrial membrane potential / vesicle-fusing ATPase / K48-linked polyubiquitin modification-dependent protein binding / regulation of aerobic respiration / retrograde protein transport, ER to cytosol / stress granule disassembly / NAD+ metabolic process / ATPase complex / regulation of synapse organization / ubiquitin-specific protease binding / ciliary tip / MHC class I protein binding / positive regulation of ATP biosynthetic process / ubiquitin-like protein ligase binding / RHOH GTPase cycle / polyubiquitin modification-dependent protein binding / autophagosome maturation / endoplasmic reticulum to Golgi vesicle-mediated transport / negative regulation of hippo signaling / HSF1 activation / interstrand cross-link repair / ATP metabolic process / translesion synthesis / endoplasmic reticulum unfolded protein response / proteasomal protein catabolic process / negative regulation of protein localization to chromatin / Protein methylation / Attachment and Entry / ERAD pathway / lipid droplet / proteasome complex / viral genome replication / Josephin domain DUBs / N-glycan trimming in the ER and Calnexin/Calreticulin cycle / negative regulation of smoothened signaling pathway / macroautophagy / establishment of protein localization / Hh mutants are degraded by ERAD / positive regulation of protein-containing complex assembly / Hedgehog ligand biogenesis / Defective CFTR causes cystic fibrosis / positive regulation of non-canonical NF-kappaB signal transduction / Translesion Synthesis by POLH / ADP binding / ABC-family proteins mediated transport / autophagy / cytoplasmic stress granule / Aggrephagy / azurophil granule lumen / positive regulation of protein catabolic process / Ovarian tumor domain proteases / KEAP1-NFE2L2 pathway / positive regulation of canonical Wnt signaling pathway / double-strand break repair / positive regulation of proteasomal ubiquitin-dependent protein catabolic process / E3 ubiquitin ligases ubiquitinate target proteins / cellular response to heat / site of double-strand break / Neddylation / secretory granule lumen / protein phosphatase binding / regulation of apoptotic process / ubiquitin-dependent protein catabolic process / ficolin-1-rich granule lumen / proteasome-mediated ubiquitin-dependent protein catabolic process / Attachment and Entry / ciliary basal body / protein ubiquitination / intracellular membrane-bounded organelle / protein domain specific binding / DNA repair / DNA damage response / ubiquitin protein ligase binding / Neutrophil degranulation / lipid binding / endoplasmic reticulum membrane / perinuclear region of cytoplasm / glutamatergic synapse / endoplasmic reticulum / ATP hydrolysis activity Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.0 Å | |||||||||
 Authors Authors | Liu S / Wang T | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: To Be Published Journal: To Be PublishedTitle: Cryo-EM structure of dodecamer P97 at 2.99 Angstroms resolution Authors: Liu S / Wang T | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_32827.map.gz emd_32827.map.gz | 945.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-32827-v30.xml emd-32827-v30.xml emd-32827.xml emd-32827.xml | 12.7 KB 12.7 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_32827.png emd_32827.png | 159.3 KB | ||
| Filedesc metadata |  emd-32827.cif.gz emd-32827.cif.gz | 5.4 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-32827 http://ftp.pdbj.org/pub/emdb/structures/EMD-32827 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-32827 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-32827 | HTTPS FTP |
-Related structure data
| Related structure data |  7wubMC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_32827.map.gz / Format: CCP4 / Size: 1000 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_32827.map.gz / Format: CCP4 / Size: 1000 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.83 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
- Sample components
Sample components
-Entire : dodecamer complex of P97
| Entire | Name: dodecamer complex of P97 |
|---|---|
| Components |
|
-Supramolecule #1: dodecamer complex of P97
| Supramolecule | Name: dodecamer complex of P97 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#3 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Transitional endoplasmic reticulum ATPase
| Macromolecule | Name: Transitional endoplasmic reticulum ATPase / type: protein_or_peptide / ID: 1 / Number of copies: 10 / Enantiomer: LEVO / EC number: vesicle-fusing ATPase |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 84.543727 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: NRPNRLIVDE AINEDNSVVS LSQPKMDELQ LFRGDTVLLK GKKRREAVCI VLSDDTCSDE KIRMNRVVRN NLRVRLGDVI SIQPCPDVK YGKRIHVLPI DDTVEGITGN LFEVYLKPYF LEAYRPIRKG DIFLVRGGMR AVEFKVVETD PSPYCIVAPD T VIHCEGEP ...String: NRPNRLIVDE AINEDNSVVS LSQPKMDELQ LFRGDTVLLK GKKRREAVCI VLSDDTCSDE KIRMNRVVRN NLRVRLGDVI SIQPCPDVK YGKRIHVLPI DDTVEGITGN LFEVYLKPYF LEAYRPIRKG DIFLVRGGMR AVEFKVVETD PSPYCIVAPD T VIHCEGEP IKREDEEESL NEVGYDDIGG CRKQLAQIKE MVELPLRHPA LFKAIGVKPP RGILLYGPPG TGKTLIARAV AN ETGAFFF LINGPEIMSK LAGESESNLR KAFEEAEKNA PAIIFIDELD AIAPKREKTH GEVERRIVSQ LLTLMDGLKQ RAH VIVMAA TNRPNSIDPA LRRFGRFDRE VDIGIPDATG RLEILQIHTK NMKLADDVDL EQVANETHGH VGADLAALCS EAAL QAIRK KMDLIDLEDE TIDAEVMNSL AVTMDDFRWA LSQSNPSALR ETVVEVPQVT WEDIGGLEDV KRELQELVQY PVEHP DKFL KFGMTPSKGV LFYGPPGCGK TLLAKAIANE CQANFISIKG PELLTMWFGE SEANVREIFD KARQAAPCVL FFDELD SIA KARGGNIGDG GGAADRVINQ ILTEMDGMST KKNVFIIGAT NRPDIIDPAI LRPGRLDQLI YIPLPDEKSR VAILKAN LR KSPVAKDVDL EFLAKMTNGF SGADLTEICQ RACKLAIRES IESEIRRERE RQTNPSAMEV EEDDPVPEIR RDHFEEAM R FARRSVSDND IRKYEMFAQT LQQSRGFGSF RFPS UniProtKB: Transitional endoplasmic reticulum ATPase |
-Macromolecule #2: Transitional endoplasmic reticulum ATPase
| Macromolecule | Name: Transitional endoplasmic reticulum ATPase / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO / EC number: vesicle-fusing ATPase |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 64.048098 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: EVGYDDIGGC RKQLAQIKEM VELPLRHPAL FKAIGVKPPR GILLYGPPGT GKTLIARAVA NETGAFFFLI NGPEIMSKLA GESESNLRK AFEEAEKNAP AIIFIDELDA IAPKREKTHG EVERRIVSQL LTLMDGLKQR AHVIVMAATN RPNSIDPALR R FGRFDREV ...String: EVGYDDIGGC RKQLAQIKEM VELPLRHPAL FKAIGVKPPR GILLYGPPGT GKTLIARAVA NETGAFFFLI NGPEIMSKLA GESESNLRK AFEEAEKNAP AIIFIDELDA IAPKREKTHG EVERRIVSQL LTLMDGLKQR AHVIVMAATN RPNSIDPALR R FGRFDREV DIGIPDATGR LEILQIHTKN MKLADDVDLE QVANETHGHV GADLAALCSE AALQAIRKKM DLIDLEDETI DA EVMNSLA VTMDDFRWAL SQSNPSALRE TVVEVPQVTW EDIGGLEDVK RELQELVQYP VEHPDKFLKF GMTPSKGVLF YGP PGCGKT LLAKAIANEC QANFISIKGP ELLTMWFGES EANVREIFDK ARQAAPCVLF FDELDSIAKA RGGNIGDGGG AADR VINQI LTEMDGMSTK KNVFIIGATN RPDIIDPAIL RPGRLDQLIY IPLPDEKSRV AILKANLRKS PVAKDVDLEF LAKMT NGFS GADLTEICQR ACKLAIRESI ESEIRRERQT QTNPSAMEVE EDDPVPEIRR DHFEEAMRFA RRSVSDNDIR KYEMFA QTL QQSRGFGSFR FPS UniProtKB: Transitional endoplasmic reticulum ATPase |
-Macromolecule #3: Transitional endoplasmic reticulum ATPase
| Macromolecule | Name: Transitional endoplasmic reticulum ATPase / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO / EC number: vesicle-fusing ATPase |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 64.105184 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: EVGYDDIGGC RKQLAQIKEM VELPLRHPAL FKAIGVKPPR GILLYGPPGT GKTLIARAVA NETGAFFFLI NGPEIMSKLA GESESNLRK AFEEAEKNAP AIIFIDELDA IAPKREKTHG EVERRIVSQL LTLMDGLKQR AHVIVMAATN RPNSIDPALR R FGRFDREV ...String: EVGYDDIGGC RKQLAQIKEM VELPLRHPAL FKAIGVKPPR GILLYGPPGT GKTLIARAVA NETGAFFFLI NGPEIMSKLA GESESNLRK AFEEAEKNAP AIIFIDELDA IAPKREKTHG EVERRIVSQL LTLMDGLKQR AHVIVMAATN RPNSIDPALR R FGRFDREV DIGIPDATGR LEILQIHTKN MKLADDVDLE QVANETHGHV GADLAALCSE AALQAIRKKM DLIDLEDETI DA EVMNSLA VTMDDFRWAL SQSNPSALRE TVVEVPQVTW EDIGGLEDVK RELQELVQYP VEHPDKFLKF GMTPSKGVLF YGP PGCGKT LLAKAIANEC QANFISIKGP ELLTMWFGES EANVREIFDK ARQAAPCVLF FDELDSIAKA RGGNIGDGGG AADR VINQI LTEMDGMSTK KNVFIIGATN RPDIIDPAIL RPGRLDQLIY IPLPDEKSRV AILKANLRKS PVAKDVDLEF LAKMT NGFS GADLTEICQR ACKLAIRESI ESEIRRERER QTNPSAMEVE EDDPVPEIRR DHFEEAMRFA RRSVSDNDIR KYEMFA QTL QQSRGFGSFR FPS UniProtKB: Transitional endoplasmic reticulum ATPase |
-Macromolecule #4: ADENOSINE-5'-DIPHOSPHATE
| Macromolecule | Name: ADENOSINE-5'-DIPHOSPHATE / type: ligand / ID: 4 / Number of copies: 12 / Formula: ADP |
|---|---|
| Molecular weight | Theoretical: 427.201 Da |
| Chemical component information |  ChemComp-ADP: |
-Macromolecule #5: 3-[3-cyclopentylsulfanyl-5-[[3-methyl-4-(4-methylsulfonylphenyl)p...
| Macromolecule | Name: 3-[3-cyclopentylsulfanyl-5-[[3-methyl-4-(4-methylsulfonylphenyl)phenoxy]methyl]-1,2,4-triazol-4-yl]pyridine type: ligand / ID: 5 / Number of copies: 12 / Formula: Y6Y |
|---|---|
| Molecular weight | Theoretical: 520.666 Da |
| Chemical component information | 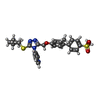 ChemComp-Y6Y: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | 3D array |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 QUANTUM (4k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: SPOT SCAN / Imaging mode: BRIGHT FIELD / Nominal defocus max: 3.0 µm / Nominal defocus min: 1.2 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: OTHER |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.0 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 157000 |
| Initial angle assignment | Type: OTHER |
| Final angle assignment | Type: OTHER |
 Movie
Movie Controller
Controller


















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)




















