+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | dmDicer2-LoqsPD-dsRNA Dimer status | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Ribonuclease / RNA BINDING PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationpositive regulation of Toll signaling pathway / : / lncRNA catabolic process / MicroRNA (miRNA) biogenesis / RNAi-mediated antiviral immune response / Small interfering RNA (siRNA) biogenesis / female germ-line stem cell asymmetric division / PKR-mediated signaling / regulation of regulatory ncRNA processing / dsRNA transport ...positive regulation of Toll signaling pathway / : / lncRNA catabolic process / MicroRNA (miRNA) biogenesis / RNAi-mediated antiviral immune response / Small interfering RNA (siRNA) biogenesis / female germ-line stem cell asymmetric division / PKR-mediated signaling / regulation of regulatory ncRNA processing / dsRNA transport / dosage compensation by hyperactivation of X chromosome / RISC complex binding / global gene silencing by mRNA cleavage / germ-line stem cell population maintenance / apoptotic DNA fragmentation / ribonuclease III / deoxyribonuclease I activity / RISC-loading complex / miRNA metabolic process / detection of virus / RISC complex assembly / regulatory ncRNA-mediated post-transcriptional gene silencing / ribonuclease III activity / pre-miRNA processing / siRNA processing / siRNA binding / positive regulation of innate immune response / ATP-dependent activity, acting on RNA / RISC complex / positive regulation of defense response to virus by host / central nervous system development / mRNA 3'-UTR binding / locomotory behavior / helicase activity / cellular response to virus / heterochromatin formation / cytoplasmic ribonucleoprotein granule / double-stranded RNA binding / defense response to virus / perinuclear region of cytoplasm / ATP hydrolysis activity / RNA binding / ATP binding / nucleus / cytosol / cytoplasm Similarity search - Function | |||||||||
| Biological species |   | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.12 Å | |||||||||
 Authors Authors | Su S / Wang J | |||||||||
| Funding support |  China, 2 items China, 2 items
| |||||||||
 Citation Citation |  Journal: Nature / Year: 2022 Journal: Nature / Year: 2022Title: Structural insights into dsRNA processing by Drosophila Dicer-2-Loqs-PD. Authors: Shichen Su / Jia Wang / Ting Deng / Xun Yuan / Jinqiu He / Nan Liu / Xiaomin Li / Ying Huang / Hong-Wei Wang / Jinbiao Ma /  Abstract: Small interfering RNAs (siRNAs) are the key components for RNA interference (RNAi), a conserved RNA-silencing mechanism in many eukaryotes. In Drosophila, an RNase III enzyme Dicer-2 (Dcr-2), aided ...Small interfering RNAs (siRNAs) are the key components for RNA interference (RNAi), a conserved RNA-silencing mechanism in many eukaryotes. In Drosophila, an RNase III enzyme Dicer-2 (Dcr-2), aided by its cofactor Loquacious-PD (Loqs-PD), has an important role in generating 21 bp siRNA duplexes from long double-stranded RNAs (dsRNAs). ATP hydrolysis by the helicase domain of Dcr-2 is critical to the successful processing of a long dsRNA into consecutive siRNA duplexes. Here we report the cryo-electron microscopy structures of Dcr-2-Loqs-PD in the apo state and in multiple states in which it is processing a 50 bp dsRNA substrate. The structures elucidated interactions between Dcr-2 and Loqs-PD, and substantial conformational changes of Dcr-2 during a dsRNA-processing cycle. The N-terminal helicase and domain of unknown function 283 (DUF283) domains undergo conformational changes after initial dsRNA binding, forming an ATP-binding pocket and a 5'-phosphate-binding pocket. The overall conformation of Dcr-2-Loqs-PD is relatively rigid during translocating along the dsRNA in the presence of ATP, whereas the interactions between the DUF283 and RIIIDb domains prevent non-specific cleavage during translocation by blocking the access of dsRNA to the RNase active centre. Additional ATP-dependent conformational changes are required to form an active dicing state and precisely cleave the dsRNA into a 21 bp siRNA duplex as confirmed by the structure in the post-dicing state. Collectively, this study revealed the molecular mechanism for the full cycle of ATP-dependent dsRNA processing by Dcr-2-Loqs-PD. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_32236.map.gz emd_32236.map.gz | 59.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-32236-v30.xml emd-32236-v30.xml emd-32236.xml emd-32236.xml | 14.4 KB 14.4 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_32236.png emd_32236.png | 117.8 KB | ||
| Filedesc metadata |  emd-32236.cif.gz emd-32236.cif.gz | 6.9 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-32236 http://ftp.pdbj.org/pub/emdb/structures/EMD-32236 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-32236 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-32236 | HTTPS FTP |
-Validation report
| Summary document |  emd_32236_validation.pdf.gz emd_32236_validation.pdf.gz | 537.5 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_32236_full_validation.pdf.gz emd_32236_full_validation.pdf.gz | 537.1 KB | Display | |
| Data in XML |  emd_32236_validation.xml.gz emd_32236_validation.xml.gz | 6.2 KB | Display | |
| Data in CIF |  emd_32236_validation.cif.gz emd_32236_validation.cif.gz | 7 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-32236 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-32236 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-32236 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-32236 | HTTPS FTP |
-Related structure data
| Related structure data |  7w0aMC  7w0bC  7w0cC  7w0dC  7w0eC  7w0fC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_32236.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_32236.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.0742 Å | ||||||||||||||||||||||||||||||||||||
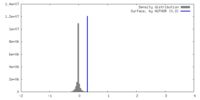
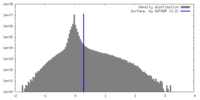
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
- Sample components
Sample components
-Entire : Dicer2-LoqsPD-dsRNA complex at its initial binding state
| Entire | Name: Dicer2-LoqsPD-dsRNA complex at its initial binding state |
|---|---|
| Components |
|
-Supramolecule #1: Dicer2-LoqsPD-dsRNA complex at its initial binding state
| Supramolecule | Name: Dicer2-LoqsPD-dsRNA complex at its initial binding state type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Dicer-2, isoform A
| Macromolecule | Name: Dicer-2, isoform A / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO / EC number: deoxyribonuclease I |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 198.006688 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MEDVEIKPRG YQLRLVDHLT KSNGIVYLPT GSGKTFVAIL VLKRFSQDFD KPIESGGKRA LFMCNTVELA RQQAMAVRRC TNFKVGFYV GEQGVDDWTR GMWSDEIKKN QVLVGTAQVF LDMVTQTYVA LSSLSVVIID ECHHGTGHHP FREFMRLFTI A NQTKLPRV ...String: MEDVEIKPRG YQLRLVDHLT KSNGIVYLPT GSGKTFVAIL VLKRFSQDFD KPIESGGKRA LFMCNTVELA RQQAMAVRRC TNFKVGFYV GEQGVDDWTR GMWSDEIKKN QVLVGTAQVF LDMVTQTYVA LSSLSVVIID ECHHGTGHHP FREFMRLFTI A NQTKLPRV VGLTGVLIKG NEITNVATKL KELEITYRGN IITVSDTKEM ENVMLYATKP TEVMVSFPHQ EQVLTVTRLI SA EIEKFYV SLDLMNIGVQ PIRRSKSLQC LRDPSKKSFV KQLFNDFLYQ MKEYGIYAAS IAIISLIVEF DIKRRQAETL SVK LMHRTA LTLCEKIRHL LVQKLQDMTY DDDDDNVNTE EVIMNFSTPK VQRFLMSLKV SFADKDPKDI CCLVFVERRY TCKC IYGLL LNYIQSTPEL RNVLTPQFMV GRNNISPDFE SVLERKWQKS AIQQFRDGNA NLMICSSVLE EGIDVQACNH VFILD PVKT FNMYVQSKGR ARTTEAKFVL FTADKEREKT IQQIYQYRKA HNDIAEYLKD RVLEKTEPEL YEIKGHFQDD IDPFTN ENG AVLLPNNALA ILHRYCQTIP TDAFGFVIPW FHVLQEDERD RIFGVSAKGK HVISINMPVN CMLRDTIYSD PMDNVKT AK ISAAFKACKV LYSLGELNER FVPKTLKERV ASIADVHFEH WNKYGDSVTA TVNKADKSKD RTYKTECPLE FYDALPRV G EICYAYEIFL EPQFESCEYT EHMYLNLQTP RNYAILLRNK LPRLAEMPLF SNQGKLHVRV ANAPLEVIIQ NSEQLELLH QFHGMVFRDI LKIWHPFFVL DRRSKENSYL VVPLILGAGE QKCFDWELMT NFRRLPQSHG SNVQQREQQP APRPEDFEGK IVTQWYANY DKPMLVTKVH RELTPLSYME KNQQDKTYYE FTMSKYGNRI GDVVHKDKFM IEVRDLTEQL TFYVHNRGKF N AKSKAKMK VILIPELCFN FNFPGDLWLK LIFLPSILNR MYFLLHAEAL RKRFNTYLNL HLLPFNGTDY MPRPLEIDYS LK RNVDPLG NVIPTEDIEE PKSLLEPMPT KSIEASVANL EITEFENPWQ KYMEPVDLSR NLLSTYPVEL DYYYHFSVGN VCE MNEMDF EDKEYWAKNQ FHMPTGNIYG NRTPAKTNAN VPALMPSKPT VRGKVKPLLI LQKTVSKEHI TPAEQGEFLA AITA SSAAD VFDMERLEIL GNSFLKLSAT LYLASKYSDW NEGTLTEVKS KLVSNRNLLF CLIDADIPKT LNTIQFTPRY TWLPP GISL PHNVLALWRE NPEFAKIIGP HNLRDLALGD EESLVKGNCS DINYNRFVEG CRANGQSFYA GADFSSEVNF CVGLVT IPN KVIADTLEAL LGVIVKNYGL QHAFKMLEYF KICRADIDKP LTQLLNLELG GKKMRANVNT TEIDGFLINH YYLEKNL GY TFKDRRYLLQ ALTHPSYPTN RITGSYQELE FIGNAILDFL ISAYIFENNT KMNPGALTDL RSALVNNTTL ACICVRHR L HFFILAENAK LSEIISKFVN FQESQGHRVT NYVRILLEEA DVQPTPLDLD DELDMTELPH ANKCISQEAE KGVPPKGEF NMSTNVDVPK ALGDVLEALI AAVYLDCRDL QRTWEVIFNL FEPELQEFTR KVPINHIRQL VEHKHAKPVF SSPIVEGETV MVSCQFTCM EKTIKVYGFG SNKDQAKLSA AKHALQQLSK CDA UniProtKB: Endoribonuclease Dcr-2 |
-Macromolecule #2: Loquacious, isoform D
| Macromolecule | Name: Loquacious, isoform D / type: protein_or_peptide / ID: 2 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 38.502574 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MDQENFHGSS LPQQLQNLHI QPQQASPNPV QTGFAPRRHY NNLVGLGNGN AVSGSPVKGA PLGQRHVKLK KEKISAQVAQ LSQPGQLQL SDVGDPALAG GSGLQGGVGL MGVILPSDEA LKFVSETDAN GLAMKTPVSI LQELLSRRGI TPGYELVQIE G AIHEPTFR ...String: MDQENFHGSS LPQQLQNLHI QPQQASPNPV QTGFAPRRHY NNLVGLGNGN AVSGSPVKGA PLGQRHVKLK KEKISAQVAQ LSQPGQLQL SDVGDPALAG GSGLQGGVGL MGVILPSDEA LKFVSETDAN GLAMKTPVSI LQELLSRRGI TPGYELVQIE G AIHEPTFR FRVSFKDKDT PFTAMGAGRS KKEAKHAAAR ALIDKLIGAQ LPESPSSSAG PSVTGLTVAG SGGDGNANAT GG GDASDKT VGNPIGWLQE MCMQRRWPPP SYETETEVGL PHERLFTIAC SILNYREMGK GKSKKIAKRL AAHRMWMRLQ ETP IDSGKI SDSICGELEG EVSIIQDIDR YEQVSKDFEF IKI UniProtKB: Protein Loquacious |
-Macromolecule #3: RNA (30-MER)
| Macromolecule | Name: RNA (30-MER) / type: rna / ID: 3 / Number of copies: 2 |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 10.014998 KDa |
| Sequence | String: AGAGACUUGG GCAAUGUGAC UGCUGAUCAG C |
-Macromolecule #4: RNA (32-MER)
| Macromolecule | Name: RNA (32-MER) / type: rna / ID: 4 / Number of copies: 2 |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 10.138027 KDa |
| Sequence | String: GCUGAUCAGC AGUCACAUUG CCCAAGUCUC UU |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.3 mg/mL |
|---|---|
| Buffer | pH: 8 |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: OTHER |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.12 Å / Resolution method: FSC 0.143 CUT-OFF / Software - Name: RELION (ver. 3.0) / Number images used: 215742 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
-Atomic model buiding 1
| Refinement | Protocol: RIGID BODY FIT |
|---|---|
| Output model |  PDB-7w0a: |
 Movie
Movie Controller
Controller











 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)