+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-30592 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structural insights into membrane remodeling by SNX1 | ||||||||||||||||||
 Map data Map data | |||||||||||||||||||
 Sample Sample |
| ||||||||||||||||||
 Keywords Keywords | Coat complex / Membrane deformation / LIPID BINDING PROTEIN / helical assembly / PROTEIN TRANSPORT | ||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationretromer, tubulation complex / lamellipodium morphogenesis / leptin receptor binding / early endosome to Golgi transport / transferrin receptor binding / epidermal growth factor receptor binding / phosphatidylinositol binding / insulin receptor binding / intracellular protein transport / receptor internalization ...retromer, tubulation complex / lamellipodium morphogenesis / leptin receptor binding / early endosome to Golgi transport / transferrin receptor binding / epidermal growth factor receptor binding / phosphatidylinositol binding / insulin receptor binding / intracellular protein transport / receptor internalization / positive regulation of protein catabolic process / lamellipodium / early endosome membrane / lysosome / protein heterodimerization activity / Golgi apparatus / protein homodimerization activity / cytosol Similarity search - Function | ||||||||||||||||||
| Biological species |  | ||||||||||||||||||
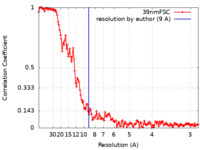
| Method | helical reconstruction / cryo EM / Resolution: 9.0 Å | ||||||||||||||||||
 Authors Authors | Zhang Y / Pang X | ||||||||||||||||||
| Funding support |  China, 5 items China, 5 items
| ||||||||||||||||||
 Citation Citation |  Journal: Proc Natl Acad Sci U S A / Year: 2021 Journal: Proc Natl Acad Sci U S A / Year: 2021Title: Structural insights into membrane remodeling by SNX1. Authors: Yan Zhang / Xiaoyun Pang / Jian Li / Jiashu Xu / Victor W Hsu / Fei Sun /   Abstract: The sorting nexin (SNX) family of proteins deform the membrane to generate transport carriers in endosomal pathways. Here, we elucidate how a prototypic member, SNX1, acts in this process. Performing ...The sorting nexin (SNX) family of proteins deform the membrane to generate transport carriers in endosomal pathways. Here, we elucidate how a prototypic member, SNX1, acts in this process. Performing cryoelectron microscopy, we find that SNX1 assembles into a protein lattice that consists of helical rows of SNX1 dimers wrapped around tubular membranes in a crosslinked fashion. We also visualize the details of this structure, which provides a molecular understanding of how various parts of SNX1 contribute to its ability to deform the membrane. Moreover, we have compared the SNX1 structure with a previously elucidated structure of an endosomal coat complex formed by retromer coupled to a SNX, which reveals how the molecular organization of the SNX in this coat complex is affected by retromer. The comparison also suggests insight into intermediary stages of assembly that results in the formation of the retromer-SNX coat complex on the membrane. | ||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_30592.map.gz emd_30592.map.gz | 54.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-30592-v30.xml emd-30592-v30.xml emd-30592.xml emd-30592.xml | 14.4 KB 14.4 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_30592_fsc.xml emd_30592_fsc.xml | 13.5 KB | Display |  FSC data file FSC data file |
| Images |  emd_30592.png emd_30592.png | 123.7 KB | ||
| Filedesc metadata |  emd-30592.cif.gz emd-30592.cif.gz | 6.6 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-30592 http://ftp.pdbj.org/pub/emdb/structures/EMD-30592 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-30592 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-30592 | HTTPS FTP |
-Related structure data
| Related structure data |  7d6dMC  7d6eC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_30592.map.gz / Format: CCP4 / Size: 59.3 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_30592.map.gz / Format: CCP4 / Size: 59.3 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. generated in cubic-lattice coordinate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.42 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : sorting nexin 1
| Entire | Name: sorting nexin 1 |
|---|---|
| Components |
|
-Supramolecule #1: sorting nexin 1
| Supramolecule | Name: sorting nexin 1 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all / Details: sorting nexin 1 in membrane-bound state |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Sorting nexin-1
| Macromolecule | Name: Sorting nexin-1 / type: protein_or_peptide / ID: 1 / Number of copies: 16 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 59.740887 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: GPLGSPEFMA SGGGGCSASE RLPPPFPGMD PESEGAAGGS EPEAGDSDTE GEDIFTGAAA ATKPQSPKKT TSLFPIKNGS KENGIHEDQ DQEPQDLFAD ATVELSLDST QNNQKTMPGK TLTSHPPQEA TNSPKPQPSY EELEEEQEDQ FDLTVGITDP E KIGDGMNA ...String: GPLGSPEFMA SGGGGCSASE RLPPPFPGMD PESEGAAGGS EPEAGDSDTE GEDIFTGAAA ATKPQSPKKT TSLFPIKNGS KENGIHEDQ DQEPQDLFAD ATVELSLDST QNNQKTMPGK TLTSHPPQEA TNSPKPQPSY EELEEEQEDQ FDLTVGITDP E KIGDGMNA YVAYKVTTQT SLPMFRSRQF AVKRRFSDFL GLYEKLSEKH SQNGFIVPPP PEKSLIGMTK VKVGKEDSSS AE FLEKRRA ALERYLQRIV NHPTMLQDPD VREFLEKEEL PRAVGTQALS GAGLLKMFNK ATDAVSKMTI KMNESDIWFE EKL QEVECE EQRLRKLHAV VETLVNHRKE LALNTALFAK SLAMLGSSED NTALSRALSQ LAEVEEKIEQ LHQEQANNDF FLLA ELLSD YIRLLAIVRA AFDQRMKTWQ RWQDAQATLQ KKRESEARLL WANKPDKLQQ AKDEITEWES RVTQYERDFE RISTV VRKE VTRFEKEKSK DFKNHVMKYL ETLLHSQQQL AKYWEAFLPE AKAIS UniProtKB: Sorting nexin-1 |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | helical reconstruction |
| Aggregation state | helical array |
- Sample preparation
Sample preparation
| Concentration | 0.9 mg/mL |
|---|---|
| Buffer | pH: 7.4 / Details: 50 mM HEPES, pH7.4, 100 mM NaCl |
| Grid | Model: Quantifoil R2/1 / Material: COPPER / Mesh: 300 / Support film - Material: CARBON / Support film - topology: HOLEY ARRAY / Pretreatment - Type: PLASMA CLEANING |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Instrument: FEI VITROBOT MARK IV Details: blot for 3.5 seconds with a blot force 2 before plunging. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON II (4k x 4k) / Detector mode: COUNTING / Digitization - Dimensions - Width: 4096 pixel / Digitization - Dimensions - Height: 4096 pixel / Digitization - Frames/image: 2-28 / Number grids imaged: 2 / Number real images: 501 / Average exposure time: 2.0 sec. / Average electron dose: 25.0 e/Å2 Details: Images were collected in movie mode at 16 frames per second |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Calibrated magnification: 59000 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Details | The temperature was kept at 300K, time step was 1 fs, and secondary structure restraints was also included. |
|---|---|
| Refinement | Space: REAL / Protocol: FLEXIBLE FIT / Target criteria: Correlation coefficient |
| Output model |  PDB-7d6d: |
 Movie
Movie Controller
Controller








 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)