[English] 日本語
 Yorodumi
Yorodumi- EMDB-29982: Cryo-EM structure of human TRPV1 in cNW11 nanodisc with POPC:POPE:POPG -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of human TRPV1 in cNW11 nanodisc with POPC:POPE:POPG | ||||||||||||||||||
 Map data Map data | |||||||||||||||||||
 Sample Sample |
| ||||||||||||||||||
 Keywords Keywords | transient receptor potential V family member 1 / TRP / apo / human / channel / TRPV1 / TRP channels / pain / membrane protein / cNW11 / nanodiscs / thermo-TRP / temperature sensation / vanilloid / POPC:POPE:POPG | ||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationchemosensory behavior / response to capsazepine / sensory perception of mechanical stimulus / peptide secretion / smooth muscle contraction involved in micturition / excitatory extracellular ligand-gated monoatomic ion channel activity / temperature-gated ion channel activity / detection of chemical stimulus involved in sensory perception of pain / fever generation / detection of temperature stimulus involved in thermoception ...chemosensory behavior / response to capsazepine / sensory perception of mechanical stimulus / peptide secretion / smooth muscle contraction involved in micturition / excitatory extracellular ligand-gated monoatomic ion channel activity / temperature-gated ion channel activity / detection of chemical stimulus involved in sensory perception of pain / fever generation / detection of temperature stimulus involved in thermoception / thermoception / cellular response to acidic pH / dendritic spine membrane / TRP channels / diet induced thermogenesis / cellular response to alkaloid / cellular response to ATP / detection of temperature stimulus involved in sensory perception of pain / intracellularly gated calcium channel activity / behavioral response to pain / calcium ion import across plasma membrane / voltage-gated calcium channel activity / extracellular ligand-gated monoatomic ion channel activity / phosphatidylinositol binding / phosphoprotein binding / calcium ion transmembrane transport / GABA-ergic synapse / lipid metabolic process / calcium channel activity / transmembrane signaling receptor activity / sensory perception of taste / cellular response to heat / protein homotetramerization / postsynaptic membrane / calmodulin binding / cell surface receptor signaling pathway / negative regulation of transcription by RNA polymerase II / ATP binding / metal ion binding / membrane / plasma membrane Similarity search - Function | ||||||||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.58 Å | ||||||||||||||||||
 Authors Authors | Neuberger A / Nadezhdin KD / Sobolevsky AI | ||||||||||||||||||
| Funding support |  United States, United States,  Germany, 5 items Germany, 5 items
| ||||||||||||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2023 Journal: Nat Commun / Year: 2023Title: Human TRPV1 structure and inhibition by the analgesic SB-366791. Authors: Arthur Neuberger / Mai Oda / Yury A Nikolaev / Kirill D Nadezhdin / Elena O Gracheva / Sviatoslav N Bagriantsev / Alexander I Sobolevsky /  Abstract: Pain therapy has remained conceptually stagnant since the opioid crisis, which highlighted the dangers of treating pain with opioids. An alternative addiction-free strategy to conventional painkiller- ...Pain therapy has remained conceptually stagnant since the opioid crisis, which highlighted the dangers of treating pain with opioids. An alternative addiction-free strategy to conventional painkiller-based treatment is targeting receptors at the origin of the pain pathway, such as transient receptor potential (TRP) ion channels. Thus, a founding member of the vanilloid subfamily of TRP channels, TRPV1, represents one of the most sought-after pain therapy targets. The need for selective TRPV1 inhibitors extends beyond pain treatment, to other diseases associated with this channel, including psychiatric disorders. Here we report the cryo-electron microscopy structures of human TRPV1 in the apo state and in complex with the TRPV1-specific nanomolar-affinity analgesic antagonist SB-366791. SB-366791 binds to the vanilloid site and acts as an allosteric hTRPV1 inhibitor. SB-366791 binding site is supported by mutagenesis combined with electrophysiological recordings and can be further explored to design new drugs targeting TRPV1 in disease conditions. | ||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_29982.map.gz emd_29982.map.gz | 59.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-29982-v30.xml emd-29982-v30.xml emd-29982.xml emd-29982.xml | 20.8 KB 20.8 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_29982.png emd_29982.png | 102.9 KB | ||
| Filedesc metadata |  emd-29982.cif.gz emd-29982.cif.gz | 7 KB | ||
| Others |  emd_29982_half_map_1.map.gz emd_29982_half_map_1.map.gz emd_29982_half_map_2.map.gz emd_29982_half_map_2.map.gz | 59.2 MB 59.2 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-29982 http://ftp.pdbj.org/pub/emdb/structures/EMD-29982 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-29982 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-29982 | HTTPS FTP |
-Related structure data
| Related structure data |  8gf9MC  8gf8C  8gfaC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_29982.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_29982.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.785 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_29982_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
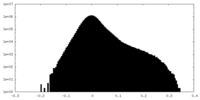
| Projections & Slices |
| ||||||||||||
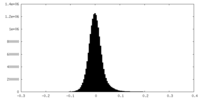
| Density Histograms |
-Half map: #1
| File | emd_29982_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
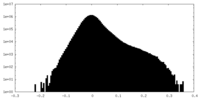
| Projections & Slices |
| ||||||||||||
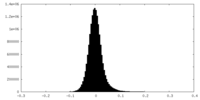
| Density Histograms |
- Sample components
Sample components
-Entire : sample 1
| Entire | Name: sample 1 |
|---|---|
| Components |
|
-Supramolecule #1: sample 1
| Supramolecule | Name: sample 1 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 94.97 KDa |
-Macromolecule #1: Transient receptor potential cation channel subfamily V member 1
| Macromolecule | Name: Transient receptor potential cation channel subfamily V member 1 type: protein_or_peptide / ID: 1 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 124.575281 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MTSKKWSSTD LGAAADPLQK DTCPDPLDGD PNSRPPPAKP QLSTAKSRTR LFGKGDSEEA FPVDCPHEEG ELDSCPTITV SPVITIQRP GDGPTGARLL SQDSVAASTE KTLRLYDRRS IFEAVAQNNC QDLESLLLFL QKSKKHLTDN EFKDPETGKT C LLKAMLNL ...String: MTSKKWSSTD LGAAADPLQK DTCPDPLDGD PNSRPPPAKP QLSTAKSRTR LFGKGDSEEA FPVDCPHEEG ELDSCPTITV SPVITIQRP GDGPTGARLL SQDSVAASTE KTLRLYDRRS IFEAVAQNNC QDLESLLLFL QKSKKHLTDN EFKDPETGKT C LLKAMLNL HDGQNTTIPL LLEIARQTDS LKELVNASYT DSYYKGQTAL HIAIERRNMA LVTLLVENGA DVQAAAHGDF FK KTKGRPG FYFGELPLSL AACTNQLGIV KFLLQNSWQT ADISARDSVG NTVLHALVEV ADNTADNTKF VTSMYNEILM LGA KLHPTL KLEELTNKKG MTPLALAAGT GKIGVLAYIL QREIQEPECR HLSRKFTEWA YGPVHSSLYD LSCIDTCEKN SVLE VIAYS SSETPNRHDM LLVEPLNRLL QDKWDRFVKR IFYFNFLVYC LYMIIFTMAA YYRPVDGLPP FKMEKTGDYF RVTGE ILSV LGGVYFFFRG IQYFLQRRPS MKTLFVDSYS EMLFFLQSLF MLATVVLYFS HLKEYVASMV FSLALGWTNM LYYTRG FQQ MGIYAVMIEK MILRDLCRFM FVYIVFLFGF STAVVTLIED GKNDSLPSES TSHRWRGPAC RPPDSSYNSL YSTCLEL FK FTIGMGDLEF TENYDFKAVF IILLLAYVIL TYILLLNMLI ALMGETVNKI AQESKNIWKL QRAITILDTE KSFLKCMR K AFRSGKLLQV GYTPDGKDDY RWCFRVDEVN WTTWNTNVGI INEDPGNCEG VKRTLSFSLR SSRVSGRHWK NFALVPLLR EASARDRQSA QPEEVYLRQF SGSLKPEDAE VFKSPAASGE KLVPRGSAAA AVSKGEELFT GVVPILVELD GDVNGHKFSV SGEGEGDAT YGKLTLKFIC TTGKLPVPWP TLVTTLTYGV QCFSRYPDHM KQHDFFKSAM PEGYVQERTI FFKDDGNYKT R AEVKFEGD TLVNRIELKG IDFKEDGNIL GHKLEYNYNS HNVYIMADKQ KNGIKVNFKI RHNIEDGSVQ LADHYQQNTP IG DGPVLLP DNHYLSTQSK LSKDPNEKRD HMVLLEFVTA AGITLGMDEL YKSGLRSWSH PQFEK UniProtKB: Transient receptor potential cation channel subfamily V member 1 |
-Macromolecule #2: (2S)-3-(hexadecanoyloxy)-2-[(9Z)-octadec-9-enoyloxy]propyl 2-(tri...
| Macromolecule | Name: (2S)-3-(hexadecanoyloxy)-2-[(9Z)-octadec-9-enoyloxy]propyl 2-(trimethylammonio)ethyl phosphate type: ligand / ID: 2 / Number of copies: 36 / Formula: POV |
|---|---|
| Molecular weight | Theoretical: 760.076 Da |
| Chemical component information |  ChemComp-POV: |
-Macromolecule #3: (2R)-3-{[(R)-hydroxy{[(1S,2R,3R,4S,5S,6R)-2,3,4,5,6-pentahydroxyc...
| Macromolecule | Name: (2R)-3-{[(R)-hydroxy{[(1S,2R,3R,4S,5S,6R)-2,3,4,5,6-pentahydroxycyclohexyl]oxy}phosphoryl]oxy}propane-1,2-diyl dioctadecanoate type: ligand / ID: 3 / Number of copies: 4 / Formula: 8IJ |
|---|---|
| Molecular weight | Theoretical: 867.138 Da |
| Chemical component information |  ChemComp-8IJ: |
-Macromolecule #4: SODIUM ION
| Macromolecule | Name: SODIUM ION / type: ligand / ID: 4 / Number of copies: 2 |
|---|---|
| Molecular weight | Theoretical: 22.99 Da |
-Macromolecule #5: water
| Macromolecule | Name: water / type: ligand / ID: 5 / Number of copies: 92 / Formula: HOH |
|---|---|
| Molecular weight | Theoretical: 18.015 Da |
| Chemical component information |  ChemComp-HOH: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 2.5 mg/mL | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 8 Component:
| ||||||||||||
| Grid | Model: UltrAuFoil R1.2/1.3 / Material: GOLD / Mesh: 300 / Support film - Material: GOLD / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE | ||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV | ||||||||||||
| Details | human TRPV1 reconstituted in cNW11 lipid nanodisc (POPC:POPE:POPG) |
- Electron microscopy
Electron microscopy
| Microscope | TFS KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Digitization - Dimensions - Width: 5760 pixel / Digitization - Dimensions - Height: 4092 pixel / Number grids imaged: 1 / Number real images: 16062 / Average exposure time: 2.0 sec. / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.0 µm / Nominal defocus min: 0.8 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Software | Name:  Coot (ver. 0.9.8.1) Coot (ver. 0.9.8.1) |
|---|---|
| Refinement | Space: REAL |
| Output model |  PDB-8gf9: |
 Movie
Movie Controller
Controller






 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)