+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Wild type P53 dimer structure from human cancer cells | ||||||||||||
 Map data Map data | P53 dimer structure | ||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | cancer / tumor suppressor / cell cycle / apoptosis / DNA repair / ANTITUMOR PROTEIN | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationLoss of function of TP53 in cancer due to loss of tetramerization ability / Regulation of TP53 Expression / signal transduction by p53 class mediator / negative regulation of G1 to G0 transition / negative regulation of glucose catabolic process to lactate via pyruvate / Transcriptional activation of cell cycle inhibitor p21 / regulation of intrinsic apoptotic signaling pathway by p53 class mediator / Activation of NOXA and translocation to mitochondria / negative regulation of pentose-phosphate shunt / ATP-dependent DNA/DNA annealing activity ...Loss of function of TP53 in cancer due to loss of tetramerization ability / Regulation of TP53 Expression / signal transduction by p53 class mediator / negative regulation of G1 to G0 transition / negative regulation of glucose catabolic process to lactate via pyruvate / Transcriptional activation of cell cycle inhibitor p21 / regulation of intrinsic apoptotic signaling pathway by p53 class mediator / Activation of NOXA and translocation to mitochondria / negative regulation of pentose-phosphate shunt / ATP-dependent DNA/DNA annealing activity / negative regulation of helicase activity / regulation of cell cycle G2/M phase transition / intrinsic apoptotic signaling pathway in response to hypoxia / regulation of fibroblast apoptotic process / oxidative stress-induced premature senescence / oligodendrocyte apoptotic process / negative regulation of miRNA processing / positive regulation of thymocyte apoptotic process / glucose catabolic process to lactate via pyruvate / regulation of tissue remodeling / positive regulation of mitochondrial membrane permeability / negative regulation of mitophagy / positive regulation of programmed necrotic cell death / mRNA transcription / bone marrow development / circadian behavior / histone deacetylase regulator activity / germ cell nucleus / regulation of mitochondrial membrane permeability involved in apoptotic process / RUNX3 regulates CDKN1A transcription / regulation of DNA damage response, signal transduction by p53 class mediator / TP53 regulates transcription of additional cell cycle genes whose exact role in the p53 pathway remain uncertain / TP53 Regulates Transcription of Death Receptors and Ligands / Activation of PUMA and translocation to mitochondria / DNA damage response, signal transduction by p53 class mediator resulting in transcription of p21 class mediator / negative regulation of glial cell proliferation / Formation of Senescence-Associated Heterochromatin Foci (SAHF) / negative regulation of neuroblast proliferation / Regulation of TP53 Activity through Association with Co-factors / mitochondrial DNA repair / T cell lineage commitment / negative regulation of DNA replication / ER overload response / B cell lineage commitment / positive regulation of cardiac muscle cell apoptotic process / thymocyte apoptotic process / TP53 regulates transcription of several additional cell death genes whose specific roles in p53-dependent apoptosis remain uncertain / TP53 Regulates Transcription of Caspase Activators and Caspases / cardiac septum morphogenesis / positive regulation of execution phase of apoptosis / entrainment of circadian clock by photoperiod / PI5P Regulates TP53 Acetylation / Association of TriC/CCT with target proteins during biosynthesis / Zygotic genome activation (ZGA) / necroptotic process / positive regulation of release of cytochrome c from mitochondria / TP53 Regulates Transcription of Genes Involved in Cytochrome C Release / TFIID-class transcription factor complex binding / rRNA transcription / mitophagy / SUMOylation of transcription factors / negative regulation of telomere maintenance via telomerase / intrinsic apoptotic signaling pathway by p53 class mediator / general transcription initiation factor binding / Transcriptional Regulation by VENTX / DNA damage response, signal transduction by p53 class mediator / response to X-ray / replicative senescence / intrinsic apoptotic signaling pathway in response to endoplasmic reticulum stress / neuroblast proliferation / cellular response to UV-C / : / hematopoietic stem cell differentiation / negative regulation of reactive oxygen species metabolic process / chromosome organization / intrinsic apoptotic signaling pathway in response to DNA damage by p53 class mediator / T cell proliferation involved in immune response / glial cell proliferation / embryonic organ development / positive regulation of RNA polymerase II transcription preinitiation complex assembly / Pyroptosis / cis-regulatory region sequence-specific DNA binding / hematopoietic progenitor cell differentiation / cellular response to glucose starvation / TP53 Regulates Transcription of Genes Involved in G1 Cell Cycle Arrest / cellular response to actinomycin D / somitogenesis / type II interferon-mediated signaling pathway / negative regulation of stem cell proliferation / core promoter sequence-specific DNA binding / positive regulation of intrinsic apoptotic signaling pathway / negative regulation of fibroblast proliferation / gastrulation / MDM2/MDM4 family protein binding / cardiac muscle cell apoptotic process / transcription initiation-coupled chromatin remodeling / 14-3-3 protein binding / mitotic G1 DNA damage checkpoint signaling / Regulation of TP53 Activity through Acetylation / response to salt stress Similarity search - Function | ||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 4.2 Å | ||||||||||||
 Authors Authors | Solares M / Kelly DF | ||||||||||||
| Funding support |  United States, 3 items United States, 3 items
| ||||||||||||
 Citation Citation |  Journal: Chembiochem / Year: 2022 Journal: Chembiochem / Year: 2022Title: High-Resolution Imaging of Human Cancer Proteins Using Microprocessor Materials. Authors: Maria J Solares / G M Jonaid / William Y Luqiu / Samantha Berry / Janki Khadela / Yanping Liang / Madison C Evans / Kevin J Pridham / William J Dearnaley / Zhi Sheng / Deborah F Kelly /  Abstract: Mutations in tumor suppressor genes, such as Tumor Protein 53 (TP53), are heavily implicated in aggressive cancers giving rise to gain- and loss-of-function phenotypes. While individual domains of ...Mutations in tumor suppressor genes, such as Tumor Protein 53 (TP53), are heavily implicated in aggressive cancers giving rise to gain- and loss-of-function phenotypes. While individual domains of the p53 protein have been studied extensively, structural information for full-length p53 remains incomplete. Functionalized microprocessor chips (microchips) with properties amenable to electron microscopy permitted us to visualize complete p53 assemblies for the first time. The new structures revealed p53 in an inactive dimeric state independent of DNA binding. Residues located at the protein-protein interface corresponded with modification sites in cancer-related hot spots. Changes in these regions may amplify the toxic effects of clinical mutations. Taken together, these results contribute advances in technology and imaging approaches to decode native protein models in different states of activation. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_28816.map.gz emd_28816.map.gz | 2.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-28816-v30.xml emd-28816-v30.xml emd-28816.xml emd-28816.xml | 17.5 KB 17.5 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_28816.png emd_28816.png | 233.1 KB | ||
| Filedesc metadata |  emd-28816.cif.gz emd-28816.cif.gz | 5.9 KB | ||
| Others |  emd_28816_half_map_1.map.gz emd_28816_half_map_1.map.gz emd_28816_half_map_2.map.gz emd_28816_half_map_2.map.gz | 2.2 MB 2.3 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-28816 http://ftp.pdbj.org/pub/emdb/structures/EMD-28816 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-28816 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-28816 | HTTPS FTP |
-Validation report
| Summary document |  emd_28816_validation.pdf.gz emd_28816_validation.pdf.gz | 762.6 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_28816_full_validation.pdf.gz emd_28816_full_validation.pdf.gz | 762.2 KB | Display | |
| Data in XML |  emd_28816_validation.xml.gz emd_28816_validation.xml.gz | 6.4 KB | Display | |
| Data in CIF |  emd_28816_validation.cif.gz emd_28816_validation.cif.gz | 7.7 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-28816 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-28816 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-28816 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-28816 | HTTPS FTP |
-Related structure data
| Related structure data |  8f2hMC  8f2iC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_28816.map.gz / Format: CCP4 / Size: 2.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_28816.map.gz / Format: CCP4 / Size: 2.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | P53 dimer structure | ||||||||||||||||||||
| Voxel size | X=Y=Z: 1.1 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: Half Map 1
| File | emd_28816_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half Map 1 | ||||||||||||
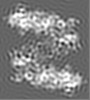
| Projections & Slices |
| ||||||||||||
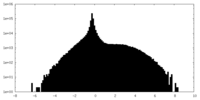
| Density Histograms |
-Half map: Half Map 2
| File | emd_28816_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half Map 2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
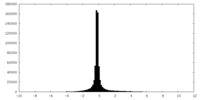
| Density Histograms |
- Sample components
Sample components
-Entire : P53 dimer isolated from U87-MG cells
| Entire | Name: P53 dimer isolated from U87-MG cells |
|---|---|
| Components |
|
-Supramolecule #1: P53 dimer isolated from U87-MG cells
| Supramolecule | Name: P53 dimer isolated from U87-MG cells / type: cell / ID: 1 / Parent: 0 / Macromolecule list: all / Details: Native protein, wild-type with no tags |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) / Organ: Brain / Tissue: Brain tumor Homo sapiens (human) / Organ: Brain / Tissue: Brain tumor |
-Macromolecule #1: Cellular tumor antigen p53
| Macromolecule | Name: Cellular tumor antigen p53 / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) / Organ: brain tumor / Tissue: brain Homo sapiens (human) / Organ: brain tumor / Tissue: brain |
| Molecular weight | Theoretical: 43.711176 KDa |
| Sequence | String: MEEPQSDPSV EPPLSQETFS DLWKLLPENN VLSPLPSQAM DDLMLSPDDI EQWFTEDPGP DEAPRMPEAA PPVAPAPAAP TPAAPAPAP SWPLSSSVPS QKTYQGSYGF RLGFLHSGTA KSVTCTYSPA LNKMFCQLAK TCPVQLWVDS TPPPGTRVRA M AIYKQSQH ...String: MEEPQSDPSV EPPLSQETFS DLWKLLPENN VLSPLPSQAM DDLMLSPDDI EQWFTEDPGP DEAPRMPEAA PPVAPAPAAP TPAAPAPAP SWPLSSSVPS QKTYQGSYGF RLGFLHSGTA KSVTCTYSPA LNKMFCQLAK TCPVQLWVDS TPPPGTRVRA M AIYKQSQH MTEVVRRCPH HERCSDSDGL APPQHLIRVE GNLRVEYLDD RNTFRHSVVV PYEPPEVGSD CTTIHYNYMC NS SCMGGMN RRPILTIITL EDSSGNLLGR NSFEVRVCAC PGRDRRTEEE NLRKKGEPHH ELPPGSTKRA LPNNTSSSPQ PKK KPLDGE YFTLQIRGRE RFEMFRELNE ALELKDAQAG KEPGGSRAHS SHLKSKKGQS TSRHKKLMFK TEGPDSD UniProtKB: Cellular tumor antigen p53 |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.2 mg/mL |
|---|---|
| Buffer | pH: 7.5 Details: 20 mM HEPES (pH 7.5), 140 mM NaCl, 2 mM MgCl2, 2 mM CaCl2, 5 mM imidazole |
| Grid | Model: Homemade / Material: SILICON NITRIDE Details: Silicon nitride chips coated with Ni-NTA layers prior to sample application |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 90 % / Chamber temperature: 298 K / Instrument: FEI VITROBOT MARK III Details: The microchip samples were loaded into a FEI Mark III Vitrobot and flash-frozen into liquid ethane.. |
| Details | Sample was placed on Silicon nitride chips coated with Ni-NTA layers |
- Electron microscopy
Electron microscopy
| Microscope | TFS TALOS F200C |
|---|---|
| Image recording | Film or detector model: FEI CETA (4k x 4k) / Number grids imaged: 10 / Number real images: 300 / Average exposure time: 1.0 sec. / Average electron dose: 5.0 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 100.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 5.0 µm / Nominal defocus min: 1.0 µm / Nominal magnification: 142000 |
| Sample stage | Specimen holder model: GATAN 626 SINGLE TILT LIQUID NITROGEN CRYO TRANSFER HOLDER Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Tecnai F20 / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: FLEXIBLE FIT / Overall B value: 50 |
|---|---|
| Output model |  PDB-8f2h: |
 Movie
Movie Controller
Controller




















 Z
Z Y
Y X
X