[English] 日本語
 Yorodumi
Yorodumi- EMDB-28651: Human S1P transporter Spns2 in an outward-facing open conformatio... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Human S1P transporter Spns2 in an outward-facing open conformation (state 4) | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | transporter / MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationregulation of eye pigmentation / regulation of humoral immune response / regulation of T cell migration / sphingolipid transporter activity / lymphocyte migration / sphingolipid biosynthetic process / Sphingolipid de novo biosynthesis / sphingosine-1-phosphate receptor signaling pathway / lipid transport / T cell homeostasis ...regulation of eye pigmentation / regulation of humoral immune response / regulation of T cell migration / sphingolipid transporter activity / lymphocyte migration / sphingolipid biosynthetic process / Sphingolipid de novo biosynthesis / sphingosine-1-phosphate receptor signaling pathway / lipid transport / T cell homeostasis / B cell homeostasis / transmembrane transporter activity / lymph node development / sensory perception of sound / bone development / endosome membrane / membrane / plasma membrane Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.47 Å | |||||||||
 Authors Authors | Ahmed S / Zhao H / Dai Y / Lee CH | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Cell / Year: 2023 Journal: Cell / Year: 2023Title: Structural and functional insights into Spns2-mediated transport of sphingosine-1-phosphate. Authors: Hongwen Chen / Shahbaz Ahmed / Hongtu Zhao / Nadia Elghobashi-Meinhardt / Yaxin Dai / Jae Hun Kim / Jeffrey G McDonald / Xiaochun Li / Chia-Hsueh Lee /   Abstract: Sphingosine-1-phosphate (S1P) is an important signaling sphingolipid that regulates the immune system, angiogenesis, auditory function, and epithelial and endothelial barrier integrity. Spinster ...Sphingosine-1-phosphate (S1P) is an important signaling sphingolipid that regulates the immune system, angiogenesis, auditory function, and epithelial and endothelial barrier integrity. Spinster homolog 2 (Spns2) is an S1P transporter that exports S1P to initiate lipid signaling cascades. Modulating Spns2 activity can be beneficial in treatments of cancer, inflammation, and immune diseases. However, the transport mechanism of Spns2 and its inhibition remain unclear. Here, we present six cryo-EM structures of human Spns2 in lipid nanodiscs, including two functionally relevant intermediate conformations that link the inward- and outward-facing states, to reveal the structural basis of the S1P transport cycle. Functional analyses suggest that Spns2 exports S1P via facilitated diffusion, a mechanism distinct from other MFS lipid transporters. Finally, we show that the Spns2 inhibitor 16d attenuates the transport activity by locking Spns2 in the inward-facing state. Our work sheds light on Spns2-mediated S1P transport and aids the development of advanced Spns2 inhibitors. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_28651.map.gz emd_28651.map.gz | 203.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-28651-v30.xml emd-28651-v30.xml emd-28651.xml emd-28651.xml | 13.3 KB 13.3 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_28651.png emd_28651.png | 63.6 KB | ||
| Filedesc metadata |  emd-28651.cif.gz emd-28651.cif.gz | 5.2 KB | ||
| Others |  emd_28651_half_map_1.map.gz emd_28651_half_map_1.map.gz emd_28651_half_map_2.map.gz emd_28651_half_map_2.map.gz | 200.6 MB 200.6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-28651 http://ftp.pdbj.org/pub/emdb/structures/EMD-28651 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-28651 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-28651 | HTTPS FTP |
-Related structure data
| Related structure data |  8ex5MC  8ex4C  8ex6C  8ex7C  8ex8C  8g92C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_28651.map.gz / Format: CCP4 / Size: 216 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_28651.map.gz / Format: CCP4 / Size: 216 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.649 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #1
| File | emd_28651_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
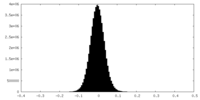
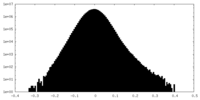
| Density Histograms |
-Half map: #2
| File | emd_28651_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
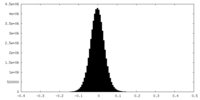
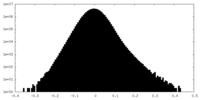
| Density Histograms |
- Sample components
Sample components
-Entire : Human S1P transporter Spns2 in an outward-facing open conformatio...
| Entire | Name: Human S1P transporter Spns2 in an outward-facing open conformation (state 4) |
|---|---|
| Components |
|
-Supramolecule #1: Human S1P transporter Spns2 in an outward-facing open conformatio...
| Supramolecule | Name: Human S1P transporter Spns2 in an outward-facing open conformation (state 4) type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Sphingosine-1-phosphate transporter SPNS2
| Macromolecule | Name: Sphingosine-1-phosphate transporter SPNS2 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 48.896184 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: EKRKAAAAIL SLGNVLNYLD RYTVAGVLLD IQQHFGVKDR GAGLLQSVFI CSFMVAAPIF GYLGDRFNRK VILSCGIFFW SAVTFSSSF IPQQYFWLLV LSRGLVGIGE ASYSTIAPTI IGDLFTKNTR TLMLSVFYFA IPLGSGLGYI TGSSVKQAAG D WHWALRVS ...String: EKRKAAAAIL SLGNVLNYLD RYTVAGVLLD IQQHFGVKDR GAGLLQSVFI CSFMVAAPIF GYLGDRFNRK VILSCGIFFW SAVTFSSSF IPQQYFWLLV LSRGLVGIGE ASYSTIAPTI IGDLFTKNTR TLMLSVFYFA IPLGSGLGYI TGSSVKQAAG D WHWALRVS PVLGMITGTL ILILVPATKR GHADQLGDQL KARTSWLRDM KALIRNRSYV FSSLATSAVS FATGALGMWI PL YLHRAQV VQKTAETCNS PPCGAKDSLI FGAITCFTGF LGVVTGAGAT RWCRLKTQRA DPLVCAVGML GSAIFICLIF VAA KSSIVG AYICIFVGET LLFSNWAITA DILMYVVIPT RRATAVALQS FTSHLLGDAG SPYLIGFISD LIRQSTKDSP LWEF LSLGY ALMLCPFVVV LGGMFFLATA LFFVSDRARA EQQVNQLAMP PASVKV UniProtKB: Sphingosine-1-phosphate transporter SPNS2 |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 65.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: OTHER / Imaging mode: BRIGHT FIELD / Nominal defocus max: 1.6 µm / Nominal defocus min: 0.6 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: OTHER |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.47 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 422542 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller










 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)