[English] 日本語
 Yorodumi
Yorodumi- EMDB-22034: Human GABAA receptor alpha1-beta2-gamma2 subtype in complex with ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-22034 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Human GABAA receptor alpha1-beta2-gamma2 subtype in complex with GABA plus etomidate | ||||||||||||||||||
 Map data Map data | GABAA receptor alpha1-beta2-gamma2 subtype in complex with GABA plus etomidate | ||||||||||||||||||
 Sample Sample |
| ||||||||||||||||||
 Keywords Keywords | Ion channel / Cys-loop receptor / pentametic ligand gated channel / GABAA receptor / MEMBRANE PROTEIN | ||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationbenzodiazepine receptor activity / GABA receptor complex / cellular response to histamine / GABA receptor activation / inner ear receptor cell development / inhibitory synapse assembly / GABA-gated chloride ion channel activity / GABA-A receptor complex / GABA-A receptor activity / innervation ...benzodiazepine receptor activity / GABA receptor complex / cellular response to histamine / GABA receptor activation / inner ear receptor cell development / inhibitory synapse assembly / GABA-gated chloride ion channel activity / GABA-A receptor complex / GABA-A receptor activity / innervation / postsynaptic specialization membrane / gamma-aminobutyric acid signaling pathway / synaptic transmission, GABAergic / adult behavior / chloride channel activity / Signaling by ERBB4 / cochlea development / chloride channel complex / dendrite membrane / chloride transmembrane transport / cytoplasmic vesicle membrane / post-embryonic development / transmitter-gated monoatomic ion channel activity involved in regulation of postsynaptic membrane potential / GABA-ergic synapse / dendritic spine / chemical synaptic transmission / postsynaptic membrane / postsynapse / axon / extracellular exosome / plasma membrane Similarity search - Function | ||||||||||||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) /  | ||||||||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.5 Å | ||||||||||||||||||
 Authors Authors | Kim JJ / Gharpure A | ||||||||||||||||||
| Funding support |  United States, 5 items United States, 5 items
| ||||||||||||||||||
 Citation Citation |  Journal: Nature / Year: 2020 Journal: Nature / Year: 2020Title: Shared structural mechanisms of general anaesthetics and benzodiazepines. Authors: Jeong Joo Kim / Anant Gharpure / Jinfeng Teng / Yuxuan Zhuang / Rebecca J Howard / Shaotong Zhu / Colleen M Noviello / Richard M Walsh / Erik Lindahl / Ryan E Hibbs /   Abstract: Most general anaesthetics and classical benzodiazepine drugs act through positive modulation of γ-aminobutyric acid type A (GABA) receptors to dampen neuronal activity in the brain. However, direct ...Most general anaesthetics and classical benzodiazepine drugs act through positive modulation of γ-aminobutyric acid type A (GABA) receptors to dampen neuronal activity in the brain. However, direct structural information on the mechanisms of general anaesthetics at their physiological receptor sites is lacking. Here we present cryo-electron microscopy structures of GABA receptors bound to intravenous anaesthetics, benzodiazepines and inhibitory modulators. These structures were solved in a lipidic environment and are complemented by electrophysiology and molecular dynamics simulations. Structures of GABA receptors in complex with the anaesthetics phenobarbital, etomidate and propofol reveal both distinct and common transmembrane binding sites, which are shared in part by the benzodiazepine drug diazepam. Structures in which GABA receptors are bound by benzodiazepine-site ligands identify an additional membrane binding site for diazepam and suggest an allosteric mechanism for anaesthetic reversal by flumazenil. This study provides a foundation for understanding how pharmacologically diverse and clinically essential drugs act through overlapping and distinct mechanisms to potentiate inhibitory signalling in the brain. | ||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_22034.map.gz emd_22034.map.gz | 10 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-22034-v30.xml emd-22034-v30.xml emd-22034.xml emd-22034.xml | 25.1 KB 25.1 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_22034.png emd_22034.png | 107.1 KB | ||
| Filedesc metadata |  emd-22034.cif.gz emd-22034.cif.gz | 7.8 KB | ||
| Others |  emd_22034_half_map_1.map.gz emd_22034_half_map_1.map.gz emd_22034_half_map_2.map.gz emd_22034_half_map_2.map.gz | 71.2 MB 71.2 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-22034 http://ftp.pdbj.org/pub/emdb/structures/EMD-22034 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-22034 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-22034 | HTTPS FTP |
-Related structure data
| Related structure data |  6x3vMC  6x3sC  6x3tC  6x3uC  6x3wC  6x3xC  6x3zC  6x40C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_22034.map.gz / Format: CCP4 / Size: 91.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_22034.map.gz / Format: CCP4 / Size: 91.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | GABAA receptor alpha1-beta2-gamma2 subtype in complex with GABA plus etomidate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
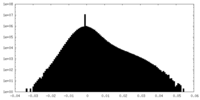
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.833 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Half map: half-volume 1
| File | emd_22034_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half-volume 1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
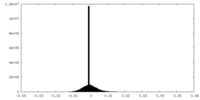
| Density Histograms |
-Half map: half-volume 2
| File | emd_22034_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half-volume 2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
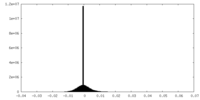
| Density Histograms |
- Sample components
Sample components
+Entire : Human GABA-A receptor alpha1-beta2-gamma2 subtype in complex with...
+Supramolecule #1: Human GABA-A receptor alpha1-beta2-gamma2 subtype in complex with...
+Supramolecule #2: Human GABA-A receptor alpha1-beta2-gamma2 subtype
+Macromolecule #1: Gamma-aminobutyric acid receptor subunit beta-2
+Macromolecule #2: Gamma-aminobutyric acid receptor subunit alpha-1
+Macromolecule #3: Gamma-aminobutyric acid receptor subunit gamma-2
+Macromolecule #4: Kappa Fab Light Chain
+Macromolecule #5: IgG2b Fab Heavy Chain
+Macromolecule #9: 2-acetamido-2-deoxy-beta-D-glucopyranose
+Macromolecule #10: GAMMA-AMINO-BUTANOIC ACID
+Macromolecule #11: Etomidate
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 8.5 mg/mL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.4 Component:
| |||||||||
| Grid | Model: Quantifoil R1.2/1.3 / Material: GOLD / Mesh: 200 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 80 sec. / Pretreatment - Atmosphere: AIR | |||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV / Details: 3.5 second blot. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Number real images: 9013 / Average electron dose: 66.07 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: AB INITIO MODEL |
|---|---|
| Output model |  PDB-6x3v: |
 Movie
Movie Controller
Controller














 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)