+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-21707 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | PRC2-AEBP2-JARID2 bound to H2AK119ub1 nucleosome | |||||||||
 Map data Map data | half map-1 for 2:1 | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | chromatin / nucleosome / transcription / cryo-EM / ubiquitination / gene regulation-DNA complex / heterochromatin / DNA binding | |||||||||
| Function / homology |  Function and homology information Function and homology informationprotein localization to pericentric heterochromatin / hepatocyte homeostasis / cellular response to trichostatin A / regulation of gliogenesis / negative regulation of striated muscle cell differentiation / regulation of kidney development / [histone H3]-lysine27 N-trimethyltransferase / response to tetrachloromethane / CAF-1 complex / histone H3K27 trimethyltransferase activity ...protein localization to pericentric heterochromatin / hepatocyte homeostasis / cellular response to trichostatin A / regulation of gliogenesis / negative regulation of striated muscle cell differentiation / regulation of kidney development / [histone H3]-lysine27 N-trimethyltransferase / response to tetrachloromethane / CAF-1 complex / histone H3K27 trimethyltransferase activity / negative regulation of keratinocyte differentiation / negative regulation of retinoic acid receptor signaling pathway / cerebellar cortex development / primary miRNA binding / random inactivation of X chromosome / regulation of adaxial/abaxial pattern formation / skeletal muscle satellite cell maintenance involved in skeletal muscle regeneration / negative regulation of cardiac muscle cell proliferation / histone H3K27 methyltransferase activity / regulatory ncRNA-mediated heterochromatin formation / sex chromatin / ubiquitin-modified histone reader activity / positive regulation of cell cycle G1/S phase transition / facultative heterochromatin formation / NuRD complex / NURF complex / regulation of cell fate specification / negative regulation of stem cell population maintenance / genomic imprinting / DNA replication-dependent chromatin assembly / Transcription of E2F targets under negative control by p107 (RBL1) and p130 (RBL2) in complex with HDAC1 / ESC/E(Z) complex / negative regulation of stem cell differentiation / regulation of stem cell differentiation / RSC-type complex / protein-lysine N-methyltransferase activity / Polo-like kinase mediated events / cardiac muscle hypertrophy in response to stress / histone H3K9me2/3 reader activity / Transcription of E2F targets under negative control by DREAM complex / chromatin silencing complex / pronucleus / positive regulation of dendrite development / G1 to G0 transition / histone H3 methyltransferase activity / histone methyltransferase activity / DNA methylation-dependent constitutive heterochromatin formation / ATPase complex / negative regulation of G1/S transition of mitotic cell cycle / spinal cord development / negative regulation of gene expression, epigenetic / lncRNA binding / G1/S-Specific Transcription / synaptic transmission, GABAergic / histone deacetylase complex / Transcriptional Regulation by E2F6 / : / Sin3-type complex / positive regulation of stem cell population maintenance / positive regulation of MAP kinase activity / histone methyltransferase complex / oligodendrocyte differentiation / RNA Polymerase I Transcription Initiation / negative regulation of transcription elongation by RNA polymerase II / G0 and Early G1 / positive regulation of protein serine/threonine kinase activity / negative regulation of cell differentiation / positive regulation of GTPase activity / cardiac muscle cell proliferation / positive regulation of epithelial to mesenchymal transition / ribonucleoprotein complex binding / subtelomeric heterochromatin formation / RNA polymerase II core promoter sequence-specific DNA binding / Cyclin E associated events during G1/S transition / pericentric heterochromatin / Transcriptional regulation of brown and beige adipocyte differentiation by EBF2 / Cyclin A:Cdk2-associated events at S phase entry / spleen development / nucleosome binding / keratinocyte differentiation / Regulation of TP53 Activity through Acetylation / protein localization to chromatin / Maturation of protein E / Maturation of protein E / ER Quality Control Compartment (ERQC) / negative regulation of cytokine production involved in inflammatory response / Myoclonic epilepsy of Lafora / FLT3 signaling by CBL mutants / Constitutive Signaling by NOTCH1 HD Domain Mutants / IRAK2 mediated activation of TAK1 complex / Prevention of phagosomal-lysosomal fusion / Alpha-protein kinase 1 signaling pathway / Glycogen synthesis / IRAK1 recruits IKK complex / IRAK1 recruits IKK complex upon TLR7/8 or 9 stimulation / Endosomal Sorting Complex Required For Transport (ESCRT) / Deposition of new CENPA-containing nucleosomes at the centromere / Membrane binding and targetting of GAG proteins / Negative regulation of FLT3 / Regulation of TBK1, IKKε (IKBKE)-mediated activation of IRF3, IRF7 Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) / | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.5 Å | |||||||||
 Authors Authors | Kasinath V / Nogales E | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Science / Year: 2021 Journal: Science / Year: 2021Title: JARID2 and AEBP2 regulate PRC2 in the presence of H2AK119ub1 and other histone modifications. Authors: Vignesh Kasinath / Curtis Beck / Paul Sauer / Simon Poepsel / Jennifer Kosmatka / Marco Faini / Daniel Toso / Ruedi Aebersold / Eva Nogales /    Abstract: Polycomb repressive complexes 1 and 2 (PRC1 and PRC2) cooperate to determine cell identity by epigenetic gene expression regulation. However, the mechanism of PRC2 recruitment by means of recognition ...Polycomb repressive complexes 1 and 2 (PRC1 and PRC2) cooperate to determine cell identity by epigenetic gene expression regulation. However, the mechanism of PRC2 recruitment by means of recognition of PRC1-mediated H2AK119ub1 remains poorly understood. Our PRC2 cryo-electron microscopy structure with cofactors JARID2 and AEBP2 bound to a H2AK119ub1-containing nucleosome reveals a bridge helix in EZH2 that connects the SET domain, H3 tail, and nucleosomal DNA. JARID2 and AEBP2 each interact with one ubiquitin and the H2A-H2B surface. JARID2 stimulates PRC2 through interactions with both the polycomb protein EED and the H2AK119-ubiquitin, whereas AEBP2 has an additional scaffolding role. The presence of these cofactors partially overcomes the inhibitory effect that H3K4me3 and H3K36me3 exert on core PRC2 (in the absence of cofactors). Our results support a key role for JARID2 and AEBP2 in the cross-talk between histone modifications and PRC2 activity. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_21707.map.gz emd_21707.map.gz | 124.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-21707-v30.xml emd-21707-v30.xml emd-21707.xml emd-21707.xml | 66.1 KB 66.1 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_21707_fsc.xml emd_21707_fsc.xml | 13.6 KB | Display |  FSC data file FSC data file |
| Images |  emd_21707.png emd_21707.png | 117.7 KB | ||
| Masks |  emd_21707_msk_1.map emd_21707_msk_1.map emd_21707_msk_2.map emd_21707_msk_2.map | 216 MB 216 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-21707.cif.gz emd-21707.cif.gz | 10.8 KB | ||
| Others |  emd_21707_additional_1.map.gz emd_21707_additional_1.map.gz emd_21707_additional_10.map.gz emd_21707_additional_10.map.gz emd_21707_additional_11.map.gz emd_21707_additional_11.map.gz emd_21707_additional_12.map.gz emd_21707_additional_12.map.gz emd_21707_additional_2.map.gz emd_21707_additional_2.map.gz emd_21707_additional_3.map.gz emd_21707_additional_3.map.gz emd_21707_additional_4.map.gz emd_21707_additional_4.map.gz emd_21707_additional_5.map.gz emd_21707_additional_5.map.gz emd_21707_additional_6.map.gz emd_21707_additional_6.map.gz emd_21707_additional_7.map.gz emd_21707_additional_7.map.gz emd_21707_additional_8.map.gz emd_21707_additional_8.map.gz emd_21707_additional_9.map.gz emd_21707_additional_9.map.gz emd_21707_half_map_1.map.gz emd_21707_half_map_1.map.gz emd_21707_half_map_2.map.gz emd_21707_half_map_2.map.gz | 125 MB 190.1 MB 189.9 MB 124.5 MB 149 MB 148.8 MB 149.1 MB 148.7 MB 124.4 MB 124.8 MB 171.4 MB 171 MB 171 MB 170.9 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-21707 http://ftp.pdbj.org/pub/emdb/structures/EMD-21707 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-21707 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-21707 | HTTPS FTP |
-Related structure data
| Related structure data |  6wkrMC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_21707.map.gz / Format: CCP4 / Size: 216 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_21707.map.gz / Format: CCP4 / Size: 216 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map-1 for 2:1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.1492 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
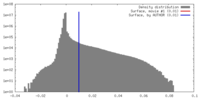
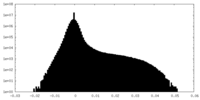
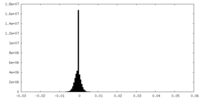
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
+Mask #1
+Mask #2
+Additional map: local res filtered map 2:1 (Bfac = 50)
+Additional map: data set 2 half 1
+Additional map: data set 2 half 2
+Additional map: data set 2 loc res filtered (bfac 50)
+Additional map: body 1 half map 1 multibody
+Additional map: body 2 half map 1 multibody
+Additional map: body 1 half map 2 multibody
+Additional map: body 2 half map 2 multibody
+Additional map: body 1 local res filtered (Bfac = 50)
+Additional map: body 2 local res filtered (Bfac = 50)
+Additional map: half map-1 for 2:1
+Additional map: half map-2 for 2:1
+Half map: half map 1 full refined map
+Half map: data set 2 half 1
- Sample components
Sample components
+Entire : PRC2-AEBP2-JARID2 bound to H2AK119ub1 nucleosome
+Supramolecule #1: PRC2-AEBP2-JARID2 bound to H2AK119ub1 nucleosome
+Supramolecule #2: PRC2-AEBP2-JARID2
+Supramolecule #3: H2AK119ub1 nucleosome
+Macromolecule #1: Ubiquitin
+Macromolecule #2: Polycomb protein SUZ12
+Macromolecule #3: Polycomb protein EED
+Macromolecule #4: Histone-binding protein RBBP4
+Macromolecule #5: Histone-lysine N-methyltransferase EZH2
+Macromolecule #6: Protein Jumonji
+Macromolecule #7: Zinc finger protein AEBP2
+Macromolecule #9: Histone H3.2
+Macromolecule #10: Histone H4
+Macromolecule #11: Histone H2A type 1
+Macromolecule #12: Histone H2B 1.1
+Macromolecule #8: DNA (314-MER)
+Macromolecule #13: MAGNESIUM ION
+Macromolecule #14: S-ADENOSYL-L-HOMOCYSTEINE
+Macromolecule #15: ZINC ION
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.9 |
|---|---|
| Grid | Model: Quantifoil R2/2 / Material: GOLD / Mesh: 300 / Support film - Material: CARBON / Support film - topology: HOLEY |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 278 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller












































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)



















































































































































 Trichoplusia ni (cabbage looper)
Trichoplusia ni (cabbage looper)

