[English] 日本語
 Yorodumi
Yorodumi- EMDB-19883: Cryo-EM Structure of Jumping Spider Rhodopsin-1 bound to a Giq he... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM Structure of Jumping Spider Rhodopsin-1 bound to a Giq heterotrimer | |||||||||||||||
 Map data Map data | ||||||||||||||||
 Sample Sample |
| |||||||||||||||
 Keywords Keywords | Opsin / GPCR / G protein / Signaling Complex / MEMBRANE PROTEIN | |||||||||||||||
| Function / homology |  Function and homology information Function and homology informationphotoreceptor activity / phototransduction / adenylate cyclase inhibitor activity / positive regulation of protein localization to cell cortex / T cell migration / Adenylate cyclase inhibitory pathway / D2 dopamine receptor binding / response to prostaglandin E / G protein-coupled serotonin receptor binding / visual perception ...photoreceptor activity / phototransduction / adenylate cyclase inhibitor activity / positive regulation of protein localization to cell cortex / T cell migration / Adenylate cyclase inhibitory pathway / D2 dopamine receptor binding / response to prostaglandin E / G protein-coupled serotonin receptor binding / visual perception / adenylate cyclase regulator activity / adenylate cyclase-inhibiting serotonin receptor signaling pathway / cellular response to forskolin / regulation of mitotic spindle organization / Regulation of insulin secretion / positive regulation of cholesterol biosynthetic process / negative regulation of insulin secretion / G protein-coupled receptor binding / G protein-coupled receptor activity / adenylate cyclase-inhibiting G protein-coupled receptor signaling pathway / response to peptide hormone / adenylate cyclase-modulating G protein-coupled receptor signaling pathway / centriolar satellite / G-protein beta/gamma-subunit complex binding / Olfactory Signaling Pathway / Activation of the phototransduction cascade / G beta:gamma signalling through PLC beta / Presynaptic function of Kainate receptors / Thromboxane signalling through TP receptor / G protein-coupled acetylcholine receptor signaling pathway / Activation of G protein gated Potassium channels / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / G-protein activation / G beta:gamma signalling through CDC42 / Prostacyclin signalling through prostacyclin receptor / Glucagon signaling in metabolic regulation / G beta:gamma signalling through BTK / Synthesis, secretion, and inactivation of Glucagon-like Peptide-1 (GLP-1) / ADP signalling through P2Y purinoceptor 12 / photoreceptor disc membrane / Glucagon-type ligand receptors / Sensory perception of sweet, bitter, and umami (glutamate) taste / GDP binding / Adrenaline,noradrenaline inhibits insulin secretion / Vasopressin regulates renal water homeostasis via Aquaporins / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / G alpha (z) signalling events / ADP signalling through P2Y purinoceptor 1 / cellular response to catecholamine stimulus / ADORA2B mediated anti-inflammatory cytokines production / G beta:gamma signalling through PI3Kgamma / adenylate cyclase-activating dopamine receptor signaling pathway / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / GPER1 signaling / G-protein beta-subunit binding / cellular response to prostaglandin E stimulus / heterotrimeric G-protein complex / G alpha (12/13) signalling events / Inactivation, recovery and regulation of the phototransduction cascade / extracellular vesicle / sensory perception of taste / Thrombin signalling through proteinase activated receptors (PARs) / signaling receptor complex adaptor activity / retina development in camera-type eye / G protein activity / GTPase binding / Ca2+ pathway / fibroblast proliferation / midbody / cell cortex / High laminar flow shear stress activates signaling by PIEZO1 and PECAM1:CDH5:KDR in endothelial cells / G alpha (i) signalling events / G alpha (s) signalling events / phospholipase C-activating G protein-coupled receptor signaling pathway / G alpha (q) signalling events / Hydrolases; Acting on acid anhydrides; Acting on GTP to facilitate cellular and subcellular movement / Ras protein signal transduction / Extra-nuclear estrogen signaling / cell population proliferation / ciliary basal body / G protein-coupled receptor signaling pathway / cell division / lysosomal membrane / GTPase activity / synapse / centrosome / GTP binding / protein-containing complex binding / nucleolus / magnesium ion binding / Golgi apparatus / signal transduction / extracellular exosome / nucleoplasm / membrane / plasma membrane / cytoplasm / cytosol Similarity search - Function | |||||||||||||||
| Biological species |  Hasarius adansoni (spider) / Hasarius adansoni (spider) /  Homo sapiens (human) Homo sapiens (human) | |||||||||||||||
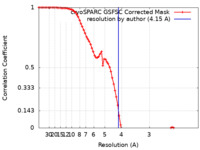
| Method | single particle reconstruction / cryo EM / Resolution: 4.15 Å | |||||||||||||||
 Authors Authors | Tejero O / Pamula F / Koyanagi M / Nagata T / Afanasyev P / Das I / Deupi X / Sheves M / Terakita A / Schertler GFX ...Tejero O / Pamula F / Koyanagi M / Nagata T / Afanasyev P / Das I / Deupi X / Sheves M / Terakita A / Schertler GFX / Rodrigues MJ / Tsai C-J | |||||||||||||||
| Funding support | European Union,  Switzerland, 4 items Switzerland, 4 items
| |||||||||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2024 Journal: Nat Commun / Year: 2024Title: Active state structures of a bistable visual opsin bound to G proteins. Authors: Oliver Tejero / Filip Pamula / Mitsumasa Koyanagi / Takashi Nagata / Pavel Afanasyev / Ishita Das / Xavier Deupi / Mordechai Sheves / Akihisa Terakita / Gebhard F X Schertler / Matthew J ...Authors: Oliver Tejero / Filip Pamula / Mitsumasa Koyanagi / Takashi Nagata / Pavel Afanasyev / Ishita Das / Xavier Deupi / Mordechai Sheves / Akihisa Terakita / Gebhard F X Schertler / Matthew J Rodrigues / Ching-Ju Tsai /     Abstract: Opsins are G protein-coupled receptors (GPCRs) that have evolved to detect light stimuli and initiate intracellular signaling cascades. Their role as signal transducers is critical to light ...Opsins are G protein-coupled receptors (GPCRs) that have evolved to detect light stimuli and initiate intracellular signaling cascades. Their role as signal transducers is critical to light perception across the animal kingdom. Opsins covalently bind to the chromophore 11-cis retinal, which isomerizes to the all-trans isomer upon photon absorption, causing conformational changes that result in receptor activation. Monostable opsins, responsible for vision in vertebrates, release the chromophore after activation and must bind another retinal molecule to remain functional. In contrast, bistable opsins, responsible for non-visual light perception in vertebrates and for vision in invertebrates, absorb a second photon in the active state to return the chromophore and protein to the inactive state. Structures of bistable opsins in the activated state have proven elusive, limiting our understanding of how they function as bidirectional photoswitches. Here we present active state structures of a bistable opsin, jumping spider rhodopsin isoform-1 (JSR1), in complex with its downstream signaling partners, the G and G heterotrimers. These structures elucidate key differences in the activation mechanisms between monostable and bistable opsins, offering essential insights for the rational engineering of bistable opsins into diverse optogenetic tools to control G protein signaling pathways. | |||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_19883.map.gz emd_19883.map.gz | 49.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-19883-v30.xml emd-19883-v30.xml emd-19883.xml emd-19883.xml | 24.1 KB 24.1 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_19883_fsc.xml emd_19883_fsc.xml | 8 KB | Display |  FSC data file FSC data file |
| Images |  emd_19883.png emd_19883.png | 60.9 KB | ||
| Filedesc metadata |  emd-19883.cif.gz emd-19883.cif.gz | 7.3 KB | ||
| Others |  emd_19883_half_map_1.map.gz emd_19883_half_map_1.map.gz emd_19883_half_map_2.map.gz emd_19883_half_map_2.map.gz | 48.9 MB 48.9 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-19883 http://ftp.pdbj.org/pub/emdb/structures/EMD-19883 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-19883 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-19883 | HTTPS FTP |
-Related structure data
| Related structure data |  9epqMC  9eppC  9eprC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_19883.map.gz / Format: CCP4 / Size: 52.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_19883.map.gz / Format: CCP4 / Size: 52.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.02 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_19883_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_19883_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
+Entire : Ternary complex of Jumping Spider Rhodopsin-1 with a chimeric Giq...
+Supramolecule #1: Ternary complex of Jumping Spider Rhodopsin-1 with a chimeric Giq...
+Supramolecule #2: Jumping Spider Rhodopsin-1
+Supramolecule #3: Human Gi/Jumping Spider Gq Chimera
+Supramolecule #4: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1
+Supramolecule #5: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2
+Macromolecule #1: Kumopsin1
+Macromolecule #2: Guanine nucleotide-binding protein G(i) subunit alpha-1
+Macromolecule #3: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1
+Macromolecule #4: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2
+Macromolecule #5: 11,20-Ethanoretinal
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 8.5 mg/mL |
|---|---|
| Buffer | pH: 6.5 |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | TFS KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 70.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.4 µm / Nominal defocus min: 1.0 µm / Nominal magnification: 165000 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller

































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)