[English] 日本語
 Yorodumi
Yorodumi- EMDB-18732: Cryo-EM structure of tetrameric human SAMHD1 State III - Relaxed -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of tetrameric human SAMHD1 State III - Relaxed | ||||||||||||
 Map data Map data | |||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | TRIPHOSPHOHYDROLASE / METALLO-ENZYME / BINUCLEAR / HD / HYDROLASE | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationNucleotide catabolism / Hydrolases; Acting on ester bonds; Triphosphoric-monoester hydrolases / deoxynucleoside triphosphate hydrolase activity / dGTP binding / dATP catabolic process / dGTPase activity / deoxyribonucleotide catabolic process / tetraspanin-enriched microdomain / dGTP catabolic process / DNA strand resection involved in replication fork processing ...Nucleotide catabolism / Hydrolases; Acting on ester bonds; Triphosphoric-monoester hydrolases / deoxynucleoside triphosphate hydrolase activity / dGTP binding / dATP catabolic process / dGTPase activity / deoxyribonucleotide catabolic process / tetraspanin-enriched microdomain / dGTP catabolic process / DNA strand resection involved in replication fork processing / negative regulation of type I interferon-mediated signaling pathway / regulation of innate immune response / somatic hypermutation of immunoglobulin genes / RNA nuclease activity / double-strand break repair via homologous recombination / Interferon alpha/beta signaling / site of double-strand break / single-stranded DNA binding / defense response to virus / protein homotetramerization / nucleic acid binding / immune response / innate immune response / DNA damage response / GTP binding / RNA binding / zinc ion binding / nucleoplasm / identical protein binding / nucleus / plasma membrane Similarity search - Function | ||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.94 Å | ||||||||||||
 Authors Authors | Acton OJ / Sheppard D / Rosenthal PB / Taylor IA | ||||||||||||
| Funding support |  United Kingdom, 3 items United Kingdom, 3 items
| ||||||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2024 Journal: Nat Commun / Year: 2024Title: Platform-directed allostery and quaternary structure dynamics of SAMHD1 catalysis. Authors: Oliver J Acton / Devon Sheppard / Simone Kunzelmann / Sarah J Caswell / Andrea Nans / Ailidh J O Burgess / Geoff Kelly / Elizabeth R Morris / Peter B Rosenthal / Ian A Taylor /  Abstract: SAMHD1 regulates cellular nucleotide homeostasis, controlling dNTP levels by catalysing their hydrolysis into 2'-deoxynucleosides and triphosphate. In differentiated CD4+ macrophage and resting T- ...SAMHD1 regulates cellular nucleotide homeostasis, controlling dNTP levels by catalysing their hydrolysis into 2'-deoxynucleosides and triphosphate. In differentiated CD4+ macrophage and resting T-cells SAMHD1 activity results in the inhibition of HIV-1 infection through a dNTP blockade. In cancer, SAMHD1 desensitizes cells to nucleoside-analogue chemotherapies. Here we employ time-resolved cryogenic-EM imaging and single-particle analysis to visualise assembly, allostery and catalysis by this multi-subunit enzyme. Our observations reveal how dynamic conformational changes in the SAMHD1 quaternary structure drive the catalytic cycle. We capture five states at high-resolution in a live catalytic reaction, revealing how allosteric activators support assembly of a stable SAMHD1 tetrameric core and how catalysis is driven by the opening and closing of active sites through pairwise coupling of active sites and order-disorder transitions in regulatory domains. This direct visualisation of enzyme catalysis dynamics within an allostery-stabilised platform sets a precedent for mechanistic studies into the regulation of multi-subunit enzymes. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_18732.map.gz emd_18732.map.gz | 2.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-18732-v30.xml emd-18732-v30.xml emd-18732.xml emd-18732.xml | 16.1 KB 16.1 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_18732_fsc.xml emd_18732_fsc.xml | 10.4 KB | Display |  FSC data file FSC data file |
| Images |  emd_18732.png emd_18732.png | 80.2 KB | ||
| Filedesc metadata |  emd-18732.cif.gz emd-18732.cif.gz | 6 KB | ||
| Others |  emd_18732_half_map_1.map.gz emd_18732_half_map_1.map.gz emd_18732_half_map_2.map.gz emd_18732_half_map_2.map.gz | 95.7 MB 95.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-18732 http://ftp.pdbj.org/pub/emdb/structures/EMD-18732 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-18732 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-18732 | HTTPS FTP |
-Validation report
| Summary document |  emd_18732_validation.pdf.gz emd_18732_validation.pdf.gz | 891.4 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_18732_full_validation.pdf.gz emd_18732_full_validation.pdf.gz | 890.9 KB | Display | |
| Data in XML |  emd_18732_validation.xml.gz emd_18732_validation.xml.gz | 18.2 KB | Display | |
| Data in CIF |  emd_18732_validation.cif.gz emd_18732_validation.cif.gz | 23.5 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-18732 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-18732 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-18732 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-18732 | HTTPS FTP |
-Related structure data
| Related structure data |  8qxmMC  8qxjC  8qxkC  8qxlC  8qxnC  8qxoC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_18732.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_18732.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.08 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_18732_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_18732_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : homotetramer of SAMHD1
| Entire | Name: homotetramer of SAMHD1 |
|---|---|
| Components |
|
-Supramolecule #1: homotetramer of SAMHD1
| Supramolecule | Name: homotetramer of SAMHD1 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Deoxynucleoside triphosphate triphosphohydrolase SAMHD1
| Macromolecule | Name: Deoxynucleoside triphosphate triphosphohydrolase SAMHD1 type: protein_or_peptide / ID: 1 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 72.305414 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MQRADSEQPS KRPRCDDSPR TPSNTPSAEA DWSPGLELHP DYKTWGPEQV CSFLRRGGFE EPVLLKNIRE NEITGALLPC LDESRFENL GVSSLGERKK LLSYIQRLVQ IHVDTMKVIN DPIHGHIELH PLLVRIIDTP QFQRLRYIKQ LGGGYYVFPG A SHNRFEHS ...String: MQRADSEQPS KRPRCDDSPR TPSNTPSAEA DWSPGLELHP DYKTWGPEQV CSFLRRGGFE EPVLLKNIRE NEITGALLPC LDESRFENL GVSSLGERKK LLSYIQRLVQ IHVDTMKVIN DPIHGHIELH PLLVRIIDTP QFQRLRYIKQ LGGGYYVFPG A SHNRFEHS LGVGYLAGCL VHALGEKQPE LQISERDVLC VQIAGLCHDL GHGPFSHMFD GRFIPLARPE VKWTHEQGSV MM FEHLINS NGIKPVMEQY GLIPEEDICF IKEQIVGPLE SPVEDSLWPY KGRPENKSFL YEIVSNKRNG IDVDKWDYFA RDC HHLGIQ NNFDYKRFIK FARVCEVDNE LRICARDKEV GNLYDMFHTR NSLHRRAYQH KVGNIIDTMI TDAFLKADDY IEIT GAGGK KYRISTAIDD MEAYTKLTDN IFLEILYSTD PKLKDAREIL KQIEYRNLFK YVGETQPTGQ IKIKREDYES LPKEV ASAK PKVLLDVKLK AEDFIVDVIN MDYGMQEKNP IDHVSFYCKT APNRAIRITK NQVSQLLPEK FAEQLIRVYC KKVDRK SLY AARQYFVQWC ADRNFTKPQD GDVIAPLITP QKKEWNDSTS VQNPTRLREA SKSRVQLFKD DPM UniProtKB: Deoxynucleoside triphosphate triphosphohydrolase SAMHD1 |
-Macromolecule #2: FE (III) ION
| Macromolecule | Name: FE (III) ION / type: ligand / ID: 2 / Number of copies: 4 / Formula: FE |
|---|---|
| Molecular weight | Theoretical: 55.845 Da |
-Macromolecule #3: MAGNESIUM ION
| Macromolecule | Name: MAGNESIUM ION / type: ligand / ID: 3 / Number of copies: 10 / Formula: MG |
|---|---|
| Molecular weight | Theoretical: 24.305 Da |
-Macromolecule #4: 2'-DEOXYADENOSINE 5'-TRIPHOSPHATE
| Macromolecule | Name: 2'-DEOXYADENOSINE 5'-TRIPHOSPHATE / type: ligand / ID: 4 / Number of copies: 4 / Formula: DTP |
|---|---|
| Molecular weight | Theoretical: 491.182 Da |
| Chemical component information |  ChemComp-DTP: |
-Macromolecule #5: GUANOSINE-5'-TRIPHOSPHATE
| Macromolecule | Name: GUANOSINE-5'-TRIPHOSPHATE / type: ligand / ID: 5 / Number of copies: 4 / Formula: GTP |
|---|---|
| Molecular weight | Theoretical: 523.18 Da |
| Chemical component information |  ChemComp-GTP: |
-Macromolecule #6: 2'-DEOXYCYTIDINE-5'-TRIPHOSPHATE
| Macromolecule | Name: 2'-DEOXYCYTIDINE-5'-TRIPHOSPHATE / type: ligand / ID: 6 / Number of copies: 2 / Formula: DCP |
|---|---|
| Molecular weight | Theoretical: 467.157 Da |
| Chemical component information |  ChemComp-DCP: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.8 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON III (4k x 4k) / Average electron dose: 33.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source: LAB6 |
| Electron optics | Illumination mode: OTHER / Imaging mode: BRIGHT FIELD / Nominal defocus max: 3.5 µm / Nominal defocus min: 1.0 µm / Nominal magnification: 75000 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: RIGID BODY FIT |
|---|---|
| Output model |  PDB-8qxm: |
 Movie
Movie Controller
Controller


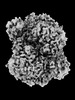






 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)