[English] 日本語
 Yorodumi
Yorodumi- EMDB-18607: Mycobacterium smegmatis inosine monophosphate dehydrogenase (IMPD... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Mycobacterium smegmatis inosine monophosphate dehydrogenase (IMPDH) ATP-bound form, compressed | |||||||||
 Map data Map data | LocScale filtered map | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Octamer / ATP complex / Purine metabolism / IMPDH / OXIDOREDUCTASE | |||||||||
| Function / homology |  Function and homology information Function and homology informationIMP dehydrogenase / IMP dehydrogenase activity / GMP biosynthetic process / GTP biosynthetic process / nucleotide binding / metal ion binding Similarity search - Function | |||||||||
| Biological species |  Mycolicibacterium smegmatis MC2 155 (bacteria) Mycolicibacterium smegmatis MC2 155 (bacteria) | |||||||||
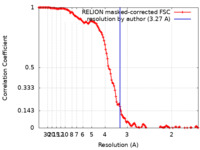
| Method | single particle reconstruction / cryo EM / Resolution: 3.27 Å | |||||||||
 Authors Authors | Bulvas O / Kouba T / Pichova I | |||||||||
| Funding support | European Union, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2024 Journal: Nat Commun / Year: 2024Title: Deciphering the allosteric regulation of mycobacterial inosine-5'-monophosphate dehydrogenase. Authors: Ondřej Bulvas / Zdeněk Knejzlík / Jakub Sýs / Anatolij Filimoněnko / Monika Čížková / Kamila Clarová / Dominik Rejman / Tomáš Kouba / Iva Pichová /  Abstract: Allosteric regulation of inosine 5'-monophosphate dehydrogenase (IMPDH), an essential enzyme of purine metabolism, contributes to the homeostasis of adenine and guanine nucleotides. However, the ...Allosteric regulation of inosine 5'-monophosphate dehydrogenase (IMPDH), an essential enzyme of purine metabolism, contributes to the homeostasis of adenine and guanine nucleotides. However, the precise molecular mechanism of IMPDH regulation in bacteria remains unclear. Using biochemical and cryo-EM approaches, we reveal the intricate molecular mechanism of the IMPDH allosteric regulation in mycobacteria. The enzyme is inhibited by both GTP and (p)ppGpp, which bind to the regulatory CBS domains and, via interactions with basic residues in hinge regions, lock the catalytic core domains in a compressed conformation. This results in occlusion of inosine monophosphate (IMP) substrate binding to the active site and, ultimately, inhibition of the enzyme. The GTP and (p)ppGpp allosteric effectors bind to their dedicated sites but stabilize the compressed octamer by a common mechanism. Inhibition is relieved by the competitive displacement of GTP or (p)ppGpp by ATP allowing IMP-induced enzyme expansion. The structural knowledge and mechanistic understanding presented here open up new possibilities for the development of allosteric inhibitors with antibacterial potential. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_18607.map.gz emd_18607.map.gz | 103.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-18607-v30.xml emd-18607-v30.xml emd-18607.xml emd-18607.xml | 19.6 KB 19.6 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_18607_fsc.xml emd_18607_fsc.xml | 13.5 KB | Display |  FSC data file FSC data file |
| Images |  emd_18607.png emd_18607.png | 120.1 KB | ||
| Masks |  emd_18607_msk_1.map emd_18607_msk_1.map | 209.3 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-18607.cif.gz emd-18607.cif.gz | 6.7 KB | ||
| Others |  emd_18607_additional_1.map.gz emd_18607_additional_1.map.gz emd_18607_half_map_1.map.gz emd_18607_half_map_1.map.gz emd_18607_half_map_2.map.gz emd_18607_half_map_2.map.gz | 195.3 MB 165.8 MB 165.8 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-18607 http://ftp.pdbj.org/pub/emdb/structures/EMD-18607 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-18607 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-18607 | HTTPS FTP |
-Related structure data
| Related structure data |  8qqwMC  8pw3C  8q65C  8qqpC  8qqqC  8qqrC  8qqtC  8qqvC  8qqxC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_18607.map.gz / Format: CCP4 / Size: 209.3 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_18607.map.gz / Format: CCP4 / Size: 209.3 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | LocScale filtered map | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.8336 Å | ||||||||||||||||||||||||||||||||||||
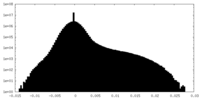
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_18607_msk_1.map emd_18607_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
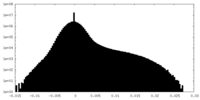
| Density Histograms |
-Additional map: RELION post-processed map
| File | emd_18607_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | RELION post-processed map | ||||||||||||
| Projections & Slices |
| ||||||||||||
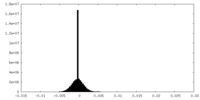
| Density Histograms |
-Half map: #2
| File | emd_18607_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_18607_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Octameric assembly of inosine monophosphate dehydrogenase in comp...
| Entire | Name: Octameric assembly of inosine monophosphate dehydrogenase in complex with ATP |
|---|---|
| Components |
|
-Supramolecule #1: Octameric assembly of inosine monophosphate dehydrogenase in comp...
| Supramolecule | Name: Octameric assembly of inosine monophosphate dehydrogenase in complex with ATP type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Mycolicibacterium smegmatis MC2 155 (bacteria) Mycolicibacterium smegmatis MC2 155 (bacteria) |
| Molecular weight | Theoretical: 426.658 KDa |
-Macromolecule #1: Inosine-5'-monophosphate dehydrogenase
| Macromolecule | Name: Inosine-5'-monophosphate dehydrogenase / type: protein_or_peptide / ID: 1 / Number of copies: 8 / Enantiomer: LEVO / EC number: IMP dehydrogenase |
|---|---|
| Source (natural) | Organism:  Mycolicibacterium smegmatis MC2 155 (bacteria) / Strain: MC2 155 Mycolicibacterium smegmatis MC2 155 (bacteria) / Strain: MC2 155 |
| Molecular weight | Theoretical: 53.388988 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MSIAESSVPI AVPVPTGGDD PTKVAMLGLT FDDVLLLPAA SDVVPATADT SSQLTKRIRL RVPLVSSAMD TVTESRMAIA MARAGGMGV LHRNLPVAEQ AGQVETVKRS EAGMVTDPVT CSPDNTLAEV DAMCARFRIS GLPVVDDTGE LVGIITNRDM R FEVDQSKP ...String: MSIAESSVPI AVPVPTGGDD PTKVAMLGLT FDDVLLLPAA SDVVPATADT SSQLTKRIRL RVPLVSSAMD TVTESRMAIA MARAGGMGV LHRNLPVAEQ AGQVETVKRS EAGMVTDPVT CSPDNTLAEV DAMCARFRIS GLPVVDDTGE LVGIITNRDM R FEVDQSKP VSEVMTKAPL ITAKEGVSAE AALGLLRRHK IEKLPIVDGH GKLTGLITVK DFVKTEQFPL STKDSDGRLL VG AAVGVGD DAWTRAMTLV DAGVDVLIVD TAHAHNRGVL DMVSRLKQAV GERVDVVGGN VATRAAAAAL VEAGADAVKV GVG PGSICT TRVVAGVGAP QITAILEAVA ACKPYGVPVI ADGGLQYSGD IAKALAAGAS TAMLGSLLAG TAESPGELIF VNGK QFKSY RGMGSLGAMQ GRGAAKSYSK DRYFQDDVLS EDKLVPEGIE GRVPFRGPLG TVIHQLTGGL RAAMGYTGSA TIEQL QQAQ FVQITAAGLK ESHPHDITMT VEAPNYYTR UniProtKB: Inosine-5'-monophosphate dehydrogenase |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 Component:
Details: 50 mM HEPES (pH 7.5), 200 mM KCl, 5 mM DTT, 4 mM MgCl2 Ligand: 2 mM ATP + 1 mM IMP | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | TFS KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Number real images: 11232 / Average exposure time: 2.0 sec. / Average electron dose: 43.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.6 µm / Nominal defocus min: 0.5 µm / Nominal magnification: 165000 |
| Sample stage | Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model | PDB ID: Chain - Source name: AlphaFold / Chain - Initial model type: in silico model |
|---|---|
| Details | Initial fitting was done in UCSF ChimeraX. Model refinement was done by iterative cycles of manual fitting with Coot and ISOLDE and automated fitting with phenix.real_space_refine. |
| Refinement | Space: REAL / Protocol: RIGID BODY FIT / Overall B value: 50.2 / Target criteria: CC coefficient |
| Output model |  PDB-8qqw: |
 Movie
Movie Controller
Controller












 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)