+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Tetracycline bound to the 30S head | ||||||||||||
 Map data Map data | |||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | Antibiotic / RIBOSOME | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationtranscription antitermination factor activity, RNA binding / transcription elongation factor complex / regulation of DNA-templated transcription elongation / transcription antitermination / maintenance of translational fidelity / ribosome biogenesis / ribosomal small subunit assembly / small ribosomal subunit / cytosolic small ribosomal subunit / cytoplasmic translation ...transcription antitermination factor activity, RNA binding / transcription elongation factor complex / regulation of DNA-templated transcription elongation / transcription antitermination / maintenance of translational fidelity / ribosome biogenesis / ribosomal small subunit assembly / small ribosomal subunit / cytosolic small ribosomal subunit / cytoplasmic translation / tRNA binding / negative regulation of translation / rRNA binding / structural constituent of ribosome / ribosome / translation / response to antibiotic / mRNA binding / RNA binding / zinc ion binding / membrane / cytosol / cytoplasm Similarity search - Function | ||||||||||||
| Biological species |  | ||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 1.82 Å | ||||||||||||
 Authors Authors | Paternoga H / Crowe-McAuliffe C / Beckert B / Wilson DN | ||||||||||||
| Funding support | European Union, 3 items
| ||||||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2023 Journal: Nat Struct Mol Biol / Year: 2023Title: Structural conservation of antibiotic interaction with ribosomes. Authors: Helge Paternoga / Caillan Crowe-McAuliffe / Lars V Bock / Timm O Koller / Martino Morici / Bertrand Beckert / Alexander G Myasnikov / Helmut Grubmüller / Jiří Nováček / Daniel N Wilson /    Abstract: The ribosome is a major target for clinically used antibiotics, but multidrug resistant pathogenic bacteria are making our current arsenal of antimicrobials obsolete. Here we present cryo-electron- ...The ribosome is a major target for clinically used antibiotics, but multidrug resistant pathogenic bacteria are making our current arsenal of antimicrobials obsolete. Here we present cryo-electron-microscopy structures of 17 distinct compounds from six different antibiotic classes bound to the bacterial ribosome at resolutions ranging from 1.6 to 2.2 Å. The improved resolution enables a precise description of antibiotic-ribosome interactions, encompassing solvent networks that mediate multiple additional interactions between the drugs and their target. Our results reveal a high structural conservation in the binding mode between antibiotics with the same scaffold, including ordered water molecules. Water molecules are visualized within the antibiotic binding sites that are preordered, become ordered in the presence of the drug and that are physically displaced on drug binding. Insight into RNA-ligand interactions will facilitate development of new antimicrobial agents, as well as other RNA-targeting therapies. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_16615.map.gz emd_16615.map.gz | 68.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-16615-v30.xml emd-16615-v30.xml emd-16615.xml emd-16615.xml | 33.7 KB 33.7 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_16615.png emd_16615.png | 84.9 KB | ||
| Filedesc metadata |  emd-16615.cif.gz emd-16615.cif.gz | 8.3 KB | ||
| Others |  emd_16615_additional_1.map.gz emd_16615_additional_1.map.gz emd_16615_half_map_1.map.gz emd_16615_half_map_1.map.gz emd_16615_half_map_2.map.gz emd_16615_half_map_2.map.gz | 1.7 GB 1.6 GB 1.6 GB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-16615 http://ftp.pdbj.org/pub/emdb/structures/EMD-16615 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-16615 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-16615 | HTTPS FTP |
-Validation report
| Summary document |  emd_16615_validation.pdf.gz emd_16615_validation.pdf.gz | 190.3 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_16615_full_validation.pdf.gz emd_16615_full_validation.pdf.gz | 189.9 KB | Display | |
| Data in XML |  emd_16615_validation.xml.gz emd_16615_validation.xml.gz | 500 B | Display | |
| Data in CIF |  emd_16615_validation.cif.gz emd_16615_validation.cif.gz | 373 B | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-16615 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-16615 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-16615 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-16615 | HTTPS FTP |
-Related structure data
| Related structure data |  8cf1MC  8ca7C  8caiC  8camC  8cazC  8cepC  8ceuC  8cf8C  8cgdC  8cgiC  8cgjC  8cgkC  8cgrC  8cguC  8cgvC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_16615.map.gz / Format: CCP4 / Size: 1.9 GB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_16615.map.gz / Format: CCP4 / Size: 1.9 GB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.681 Å | ||||||||||||||||||||||||||||||||||||
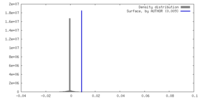
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: #1
| File | emd_16615_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
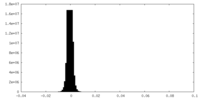
| Density Histograms |
-Half map: #2
| File | emd_16615_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
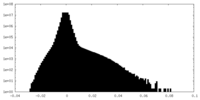
| Density Histograms |
-Half map: #1
| File | emd_16615_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
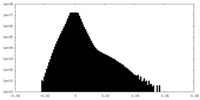
| Density Histograms |
- Sample components
Sample components
+Entire : 70S ribosomes with antibiotic cocktail
+Supramolecule #1: 70S ribosomes with antibiotic cocktail
+Macromolecule #1: 16S rRNA
+Macromolecule #2: 30S ribosomal protein S2
+Macromolecule #3: Small ribosomal subunit protein uS3
+Macromolecule #4: Small ribosomal subunit protein uS5
+Macromolecule #5: 30S ribosomal protein S7
+Macromolecule #6: Small ribosomal subunit protein uS9
+Macromolecule #7: Small ribosomal subunit protein uS10
+Macromolecule #8: Small ribosomal subunit protein uS13
+Macromolecule #9: Small ribosomal subunit protein uS14
+Macromolecule #10: Small ribosomal subunit protein uS19
+Macromolecule #11: TETRACYCLINE
+Macromolecule #12: MAGNESIUM ION
+Macromolecule #13: POTASSIUM ION
+Macromolecule #14: water
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 Component:
| |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grid | Model: Quantifoil R1.2/1.3 / Material: GOLD / Mesh: 300 / Support film - Material: GRAPHENE | |||||||||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277.15 K |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON IV (4k x 4k) / Detector mode: COUNTING / Average exposure time: 4.5 sec. / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 1.0 µm / Nominal defocus min: 0.4 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: PDB ENTRY PDB model - PDB ID: |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 1.82 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 419159 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller





















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)