[English] 日本語
 Yorodumi
Yorodumi- EMDB-16376: CryoEM structure of a tungsten-containing aldehyde oxidoreductase... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | CryoEM structure of a tungsten-containing aldehyde oxidoreductase from Aromatoleum aromaticum | ||||||||||||
 Map data Map data | |||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | Tungsten-containing enzyme / nanowires. / OXIDOREDUCTASE | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationOxidoreductases; Acting on the aldehyde or oxo group of donors; With an iron-sulfur protein as acceptor / oxidoreductase activity, acting on the aldehyde or oxo group of donors, iron-sulfur protein as acceptor / 4 iron, 4 sulfur cluster binding / oxidoreductase activity / electron transfer activity / metal ion binding Similarity search - Function | ||||||||||||
| Biological species |  Aromatoleum aromaticum (bacteria) Aromatoleum aromaticum (bacteria) | ||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.22 Å | ||||||||||||
 Authors Authors | Winiarska A / Ramirez-Amador F / Hege D / Gemmecker Y / Prinz S / Hochberg G / Heider J / Szaleniec M / Schuller JM | ||||||||||||
| Funding support | European Union,  Poland, Poland,  Germany, 3 items Germany, 3 items
| ||||||||||||
 Citation Citation |  Journal: Sci Adv / Year: 2023 Journal: Sci Adv / Year: 2023Title: A bacterial tungsten-containing aldehyde oxidoreductase forms an enzymatic decorated protein nanowire. Authors: Agnieszka Winiarska / Fidel Ramírez-Amador / Dominik Hege / Yvonne Gemmecker / Simone Prinz / Georg Hochberg / Johann Heider / Maciej Szaleniec / Jan Michael Schuller /   Abstract: Aldehyde oxidoreductases (AORs) are tungsten enzymes catalyzing the oxidation of many different aldehydes to the corresponding carboxylic acids. In contrast to other known AORs, the enzyme from the ...Aldehyde oxidoreductases (AORs) are tungsten enzymes catalyzing the oxidation of many different aldehydes to the corresponding carboxylic acids. In contrast to other known AORs, the enzyme from the denitrifying betaproteobacterium (AOR) consists of three different subunits (AorABC) and uses nicotinamide adenine dinucleotide (NAD) as an electron acceptor. Here, we reveal that the enzyme forms filaments of repeating AorAB protomers that are capped by a single NAD-binding AorC subunit, based on solving its structure via cryo-electron microscopy. The polyferredoxin-like subunit AorA oligomerizes to an electron-conducting nanowire that is decorated with enzymatically active and W-cofactor (W-co) containing AorB subunits. Our structure further reveals the binding mode of the native substrate benzoate in the AorB active site. This, together with quantum mechanics:molecular mechanics (QM:MM)-based modeling for the coordination of the W-co, enables formulation of a hypothetical catalytic mechanism that paves the way to further engineering for applications in synthetic biology and biotechnology. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_16376.map.gz emd_16376.map.gz | 59.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-16376-v30.xml emd-16376-v30.xml emd-16376.xml emd-16376.xml | 25.7 KB 25.7 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_16376.png emd_16376.png | 79.7 KB | ||
| Masks |  emd_16376_msk_1.map emd_16376_msk_1.map | 64 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-16376.cif.gz emd-16376.cif.gz | 8 KB | ||
| Others |  emd_16376_half_map_1.map.gz emd_16376_half_map_1.map.gz emd_16376_half_map_2.map.gz emd_16376_half_map_2.map.gz | 59.4 MB 59.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-16376 http://ftp.pdbj.org/pub/emdb/structures/EMD-16376 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-16376 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-16376 | HTTPS FTP |
-Related structure data
| Related structure data |  8c0zMC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_16376.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_16376.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_16376_msk_1.map emd_16376_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_16376_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_16376_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Tungsten-containing aldehyde oxidoreductase from Aromatoleum arom...
| Entire | Name: Tungsten-containing aldehyde oxidoreductase from Aromatoleum aromaticum |
|---|---|
| Components |
|
-Supramolecule #1: Tungsten-containing aldehyde oxidoreductase from Aromatoleum arom...
| Supramolecule | Name: Tungsten-containing aldehyde oxidoreductase from Aromatoleum aromaticum type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#3 |
|---|---|
| Source (natural) | Organism:  Aromatoleum aromaticum (bacteria) Aromatoleum aromaticum (bacteria) |
| Molecular weight | Theoretical: 304 KDa |
-Macromolecule #1: Aldehyde:ferredoxin oxidoreductase,tungsten-containing
| Macromolecule | Name: Aldehyde:ferredoxin oxidoreductase,tungsten-containing type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO EC number: Oxidoreductases; Acting on the aldehyde or oxo group of donors; With an iron-sulfur protein as acceptor |
|---|---|
| Source (natural) | Organism:  Aromatoleum aromaticum (bacteria) Aromatoleum aromaticum (bacteria) |
| Molecular weight | Theoretical: 65.954648 KDa |
| Recombinant expression | Organism:  Aromatoleum evansii (bacteria) Aromatoleum evansii (bacteria) |
| Sequence | String: MGWNRKVLRV NLAEGTCTPE PLNMQWADEY LGSRGLATKY LVSETDPKVD PLSPDNKMIM ATGPLTGTMA STGGRYTVVT KGPLTGAIA CSNSGGFFGA EMKFAGWDMV IFEGRSPTPV YLFIENERAE LRDASYLWGR SCWETEESIR AQHQDPLIRV S SIGRAGEN ...String: MGWNRKVLRV NLAEGTCTPE PLNMQWADEY LGSRGLATKY LVSETDPKVD PLSPDNKMIM ATGPLTGTMA STGGRYTVVT KGPLTGAIA CSNSGGFFGA EMKFAGWDMV IFEGRSPTPV YLFIENERAE LRDASYLWGR SCWETEESIR AQHQDPLIRV S SIGRAGEN QVMFACIVND LHRAAGRSGV GAVMGSKNLK AVAIRGTKGV SGIRDFPGFV RATSEAKKVL AGNPVTSEGL PK FGTQVLM NVINEMGALP TRNHRDVQFE DASKISAEAM HEKRPSDGKP QLVTNAACFG CTIACGRISA IDKTHFTVKN NPK YWGASG GLEYEAAWAL GAANGVGDLE ALQYANLLCN EQGMDPISFG ATVGAAMELY ETGVLTKERI GLDAPFGSAD ALAK LAEMT ATGEGFGKEI GLGSKRLCEK YGHPELSMSV KGQEFPAYDS RGIQGMGLAY ATSNRGACHL RGYTVASEVL GVPVK TDPH VIEGKAELVK AFQDATAVFD SAGICVFTSF AWTLADVQPQ IAAACDGDWS MDKLATVGER IWNMERQFNN AAGLGA QDD NLPPRLTSEP AKSGPAKGMV NRLAEMLPEY YGVRGWTPEG TPTPETLSRL GLS UniProtKB: Aldehyde:ferredoxin oxidoreductase,tungsten-containing |
-Macromolecule #2: Iron-sulfur cluster-binding protein potential subunit of aldehyde...
| Macromolecule | Name: Iron-sulfur cluster-binding protein potential subunit of aldehyde oxidoreductase type: protein_or_peptide / ID: 2 Details: AorA (chain C,F in the model) is fused to a N-terminal Twin-Strep-tag (two Strep-tags linked by a glycin/serin peptide) for purification purposes. Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Aromatoleum aromaticum (bacteria) Aromatoleum aromaticum (bacteria) |
| Molecular weight | Theoretical: 20.524352 KDa |
| Recombinant expression | Organism:  Aromatoleum evansii (bacteria) Aromatoleum evansii (bacteria) |
| Sequence | String: MASAWSHPQF EKGGGSGGGS GGSAWSHPQF EKSGMWKSLH IDPAKCTGCL QCEMACSYEH TGVINPSKSR IKVFSFEHEG RKVPYTCTQ CTEAWCLHSC PVDAIRLDLT TGAKMVFEDT CVGCKVCTIA CPFGTINYNQ DTGKVQKCDL CEGDPACAKA C PTAAITYI ...String: MASAWSHPQF EKGGGSGGGS GGSAWSHPQF EKSGMWKSLH IDPAKCTGCL QCEMACSYEH TGVINPSKSR IKVFSFEHEG RKVPYTCTQ CTEAWCLHSC PVDAIRLDLT TGAKMVFEDT CVGCKVCTIA CPFGTINYNQ DTGKVQKCDL CEGDPACAKA C PTAAITYI DADWTGLARM QAWAAKANTP ASAA UniProtKB: Iron-sulfur cluster-binding protein potential subunit of aldehyde oxidoreductase |
-Macromolecule #3: Similar to ferredoxin:NADH oxidoreductases or NADH oxidases,poten...
| Macromolecule | Name: Similar to ferredoxin:NADH oxidoreductases or NADH oxidases,potential subunit of aldehyde oxidoreductase type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Aromatoleum aromaticum (bacteria) Aromatoleum aromaticum (bacteria) |
| Molecular weight | Theoretical: 45.798395 KDa |
| Recombinant expression | Organism:  Aromatoleum evansii (bacteria) Aromatoleum evansii (bacteria) |
| Sequence | String: MKHVILGNGP AGVIAAETLR RAAPTDDILL FGSEDAPPYS RMAIPYLLEG NIDESGTWLR KSPGHFDRLR IHEMRGRAVS LDSERRRIL FDDGHFESWD RLLIATGSHP VRPPIPGIDL PEVQTCWTLE DARAIARFAT PGARVLQLGA GFIGCIIMEA L AARGVELT ...String: MKHVILGNGP AGVIAAETLR RAAPTDDILL FGSEDAPPYS RMAIPYLLEG NIDESGTWLR KSPGHFDRLR IHEMRGRAVS LDSERRRIL FDDGHFESWD RLLIATGSHP VRPPIPGIDL PEVQTCWTLE DARAIARFAT PGARVLQLGA GFIGCIIMEA L AARGVELT VVEMGDRMVP RMMTPTAGGM IRKWVEDQGV RVVTNAGVSR IDCRASNDAP LDVTLSTGEV VVADLVIVAA GV APNIAFL EATPVHVAKG VLVDDRLQTS VPGIFAAGDV AEAPDLFTGA HLVAAIQPNA ADQARVAALN MAGHEARLKG VLA INVLDT LGLISSSFGQ WWGEERERGG AGVEHVDEAA YRYLSLQFKD DVLIGATSIG LTEHVGALRG LIHGRVRLGE WKER LLHSP LQFVDAYIAR SQQPMALVR UniProtKB: Similar to ferredoxin:NADH oxidoreductases or NADH oxidases,potential subunit of aldehyde oxidoreductase |
-Macromolecule #4: IRON/SULFUR CLUSTER
| Macromolecule | Name: IRON/SULFUR CLUSTER / type: ligand / ID: 4 / Number of copies: 10 / Formula: SF4 |
|---|---|
| Molecular weight | Theoretical: 351.64 Da |
| Chemical component information |  ChemComp-FS1: |
-Macromolecule #5: tungsten cofactor
| Macromolecule | Name: tungsten cofactor / type: ligand / ID: 5 / Number of copies: 2 / Formula: T7R |
|---|---|
| Molecular weight | Theoretical: 1.002511 KDa |
| Chemical component information |  ChemComp-T7R: |
-Macromolecule #6: MAGNESIUM ION
| Macromolecule | Name: MAGNESIUM ION / type: ligand / ID: 6 / Number of copies: 2 / Formula: MG |
|---|---|
| Molecular weight | Theoretical: 24.305 Da |
-Macromolecule #7: BENZOIC ACID
| Macromolecule | Name: BENZOIC ACID / type: ligand / ID: 7 / Number of copies: 2 / Formula: BEZ |
|---|---|
| Molecular weight | Theoretical: 122.121 Da |
| Chemical component information | 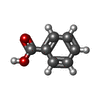 ChemComp-BEZ: |
-Macromolecule #8: FLAVIN-ADENINE DINUCLEOTIDE
| Macromolecule | Name: FLAVIN-ADENINE DINUCLEOTIDE / type: ligand / ID: 8 / Number of copies: 1 / Formula: FAD |
|---|---|
| Molecular weight | Theoretical: 785.55 Da |
| Chemical component information |  ChemComp-FAD: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.70 mg/mL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.5 Component:
| |||||||||
| Grid | Model: Quantifoil R2/1 / Material: COPPER / Mesh: 200 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 25 sec. / Details: 15 mA | |||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | TFS GLACIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON III (4k x 4k) / Number real images: 896 / Average electron dose: 40.0 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: OTHER / Cs: 2.7 mm / Nominal defocus max: 2.0 µm / Nominal defocus min: 0.5 µm |
 Movie
Movie Controller
Controller












 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)