[English] 日本語
 Yorodumi
Yorodumi- EMDB-16122: Cryo-EM structure of the wild-type solitary ECF module in MSP2N2 ... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of the wild-type solitary ECF module in MSP2N2 lipid nanodiscs in the ATPase open and nucleotide-free conformation | ||||||||||||||||||
 Map data Map data | ATPase open and nucleotide-free conformation of the wild-type solitary ECF module in MSP2N2 lipid nanodiscs at 3.8 A resolution sharpened at -148 A^2. | ||||||||||||||||||
 Sample Sample |
| ||||||||||||||||||
 Keywords Keywords | ABC Transporter / ECF transporter complex / motor / membrane protein | ||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationTranslocases / Hydrolases; Acting on acid anhydrides; Acting on acid anhydrides to catalyse transmembrane movement of substances / transmembrane transporter activity / ATPase-coupled transmembrane transporter activity / ATP-binding cassette (ABC) transporter complex / ATP hydrolysis activity / ATP binding / plasma membrane Similarity search - Function | ||||||||||||||||||
| Biological species |  Lactobacillus delbrueckii subsp. bulgaricus ATCC 11842 = JCM 1002 (bacteria) Lactobacillus delbrueckii subsp. bulgaricus ATCC 11842 = JCM 1002 (bacteria) | ||||||||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.8 Å | ||||||||||||||||||
 Authors Authors | Thangaratnarajah C / Rheinberger J / Paulino C / Slotboom DJ | ||||||||||||||||||
| Funding support | European Union,  Netherlands, 5 items Netherlands, 5 items
| ||||||||||||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2023 Journal: Nat Commun / Year: 2023Title: Expulsion mechanism of the substrate-translocating subunit in ECF transporters. Authors: Chancievan Thangaratnarajah / Mark Nijland / Luís Borges-Araújo / Aike Jeucken / Jan Rheinberger / Siewert J Marrink / Paulo C T Souza / Cristina Paulino / Dirk J Slotboom /    Abstract: Energy-coupling factor (ECF)-type transporters mediate the uptake of micronutrients in many bacteria. They consist of a substrate-translocating subunit (S-component) and an ATP-hydrolysing motor (ECF ...Energy-coupling factor (ECF)-type transporters mediate the uptake of micronutrients in many bacteria. They consist of a substrate-translocating subunit (S-component) and an ATP-hydrolysing motor (ECF module) Previous data indicate that the S-component topples within the membrane to alternately expose the binding site to either side of the membrane. In many ECF transporters, the substrate-free S-component can be expelled from the ECF module. Here we study this enigmatic expulsion step by cryogenic electron microscopy and reveal that ATP induces a concave-to-convex shape change of two long helices in the motor, thereby destroying the S-component's docking site and allowing for its dissociation. We show that adaptation of the membrane morphology to the conformational state of the motor may favour expulsion of the substrate-free S-component when ATP is bound and docking of the substrate-loaded S-component after hydrolysis. Our work provides a picture of bilayer-assisted chemo-mechanical coupling in the transport cycle of ECF transporters. | ||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_16122.map.gz emd_16122.map.gz | 59.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-16122-v30.xml emd-16122-v30.xml emd-16122.xml emd-16122.xml | 24.6 KB 24.6 KB | Display Display |  EMDB header EMDB header |
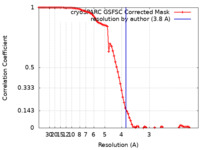
| FSC (resolution estimation) |  emd_16122_fsc.xml emd_16122_fsc.xml | 8.5 KB | Display |  FSC data file FSC data file |
| Images |  emd_16122.png emd_16122.png | 105.8 KB | ||
| Masks |  emd_16122_msk_1.map emd_16122_msk_1.map | 64 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-16122.cif.gz emd-16122.cif.gz | 7.1 KB | ||
| Others |  emd_16122_additional_1.map.gz emd_16122_additional_1.map.gz emd_16122_half_map_1.map.gz emd_16122_half_map_1.map.gz emd_16122_half_map_2.map.gz emd_16122_half_map_2.map.gz | 56.6 MB 59.3 MB 59.3 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-16122 http://ftp.pdbj.org/pub/emdb/structures/EMD-16122 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-16122 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-16122 | HTTPS FTP |
-Validation report
| Summary document |  emd_16122_validation.pdf.gz emd_16122_validation.pdf.gz | 938.5 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_16122_full_validation.pdf.gz emd_16122_full_validation.pdf.gz | 938 KB | Display | |
| Data in XML |  emd_16122_validation.xml.gz emd_16122_validation.xml.gz | 16.4 KB | Display | |
| Data in CIF |  emd_16122_validation.cif.gz emd_16122_validation.cif.gz | 21.3 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-16122 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-16122 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-16122 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-16122 | HTTPS FTP |
-Related structure data
| Related structure data |  8bmrMC  8bmpC  8bmqC  8bmsC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_16122.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_16122.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | ATPase open and nucleotide-free conformation of the wild-type solitary ECF module in MSP2N2 lipid nanodiscs at 3.8 A resolution sharpened at -148 A^2. | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.022 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_16122_msk_1.map emd_16122_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Sharpened map obtained with DeepEMhancer used for model...
| File | emd_16122_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Sharpened map obtained with DeepEMhancer used for model building and figures. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map 2 used during refinement and FSC...
| File | emd_16122_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map 2 used during refinement and FSC gold-standard resolution calculation. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map 1 used during refinement and FSC...
| File | emd_16122_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map 1 used during refinement and FSC gold-standard resolution calculation. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Wild-type solitary ECF module
| Entire | Name: Wild-type solitary ECF module |
|---|---|
| Components |
|
-Supramolecule #1: Wild-type solitary ECF module
| Supramolecule | Name: Wild-type solitary ECF module / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Lactobacillus delbrueckii subsp. bulgaricus ATCC 11842 = JCM 1002 (bacteria) Lactobacillus delbrueckii subsp. bulgaricus ATCC 11842 = JCM 1002 (bacteria) |
-Macromolecule #1: Energy-coupling factor transporter ATP-binding protein EcfA1
| Macromolecule | Name: Energy-coupling factor transporter ATP-binding protein EcfA1 type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO / EC number: Translocases |
|---|---|
| Source (natural) | Organism:  Lactobacillus delbrueckii subsp. bulgaricus ATCC 11842 = JCM 1002 (bacteria) Lactobacillus delbrueckii subsp. bulgaricus ATCC 11842 = JCM 1002 (bacteria)Strain: ATCC 11842 / DSM 20081 / BCRC 10696 / JCM 1002 / NBRC 13953 / NCIMB 11778 / NCTC 12712 / WDCM 00102 / Lb 14 |
| Molecular weight | Theoretical: 30.801861 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: GSDNIISFDH VTFTYPDSPR PALSDLSFAI ERGSWTALIG HNGSGKSTVS KLINGLLAPD DLDKSSITVD GVKLGADTVW EVREKVGIV FQNPDNQFVG ATVSDDVAFG LENRAVPRPE MLKIVAQAVA DVGMADYADS EPSNLSGGQK QRVAIAGILA V KPQVIILD ...String: GSDNIISFDH VTFTYPDSPR PALSDLSFAI ERGSWTALIG HNGSGKSTVS KLINGLLAPD DLDKSSITVD GVKLGADTVW EVREKVGIV FQNPDNQFVG ATVSDDVAFG LENRAVPRPE MLKIVAQAVA DVGMADYADS EPSNLSGGQK QRVAIAGILA V KPQVIILD ESTSMLDPEG KEQILDLVRK IKEDNNLTVI SITHDLEEAA GADQVLVLDD GQLLDQGKPE EIFPKVEMLK RI GLDIPFV YRLKQLLKER GIVLPDEIDD DEKLVQSLWQ LNSKM UniProtKB: Energy-coupling factor transporter ATP-binding protein EcfA1 |
-Macromolecule #2: Energy-coupling factor transporter ATP-binding protein EcfA2
| Macromolecule | Name: Energy-coupling factor transporter ATP-binding protein EcfA2 type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO EC number: Hydrolases; Acting on acid anhydrides; Acting on acid anhydrides to catalyse transmembrane movement of substances |
|---|---|
| Source (natural) | Organism:  Lactobacillus delbrueckii subsp. bulgaricus ATCC 11842 = JCM 1002 (bacteria) Lactobacillus delbrueckii subsp. bulgaricus ATCC 11842 = JCM 1002 (bacteria)Strain: ATCC 11842 / DSM 20081 / JCM 1002 / NBRC 13953 / NCIMB 11778 |
| Molecular weight | Theoretical: 31.672156 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MAIKFENVSY VYSPGSPLEA IGLDQLNFSL EEGKFIALVG HTGSGKSTLM QHFNALLKPT SGKIEIAGYT ITPETGNKGL KDLRRKVSL AFQFSEAQLF ENTVLKDVEY GPRNFGFSED EAREAALKWL KKVGLKDDLI EHSPFDLSGG QMRRVALAGV L AYEPEIIC ...String: MAIKFENVSY VYSPGSPLEA IGLDQLNFSL EEGKFIALVG HTGSGKSTLM QHFNALLKPT SGKIEIAGYT ITPETGNKGL KDLRRKVSL AFQFSEAQLF ENTVLKDVEY GPRNFGFSED EAREAALKWL KKVGLKDDLI EHSPFDLSGG QMRRVALAGV L AYEPEIIC LDEPAAGLDP MGRLEMMQLF KDYQAAGHTV ILVTHNMDDV ADYADDVLAL EHGRLIKHAS PKEVFKDSEW LQ KHHLAEP RSARFAAKLE AAGLKLPGQP LTMPELADAI KQSLKGGEHE UniProtKB: Energy-coupling factor transporter ATP-binding protein EcfA2 |
-Macromolecule #3: Energy-coupling factor transporter transmembrane protein EcfT
| Macromolecule | Name: Energy-coupling factor transporter transmembrane protein EcfT type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Lactobacillus delbrueckii subsp. bulgaricus ATCC 11842 = JCM 1002 (bacteria) Lactobacillus delbrueckii subsp. bulgaricus ATCC 11842 = JCM 1002 (bacteria)Strain: ATCC 11842 / DSM 20081 / JCM 1002 / NBRC 13953 / NCIMB 11778 |
| Molecular weight | Theoretical: 30.290283 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MSKIIIGRYL PGTTFVYRVD PRAKLLTTFY FIIMIFLANN WVSYLVISIF GLAYVFATGL KARVFWDGVK PMIWMIVFTS LLQTFFMAG GKVYWHWWIF TLSSEGLING LYVFIRFAMI ILVSTVMTVT TKPLEIADAM EWMLTPLKLF KVNVGMISLV I SIALRFVP ...String: MSKIIIGRYL PGTTFVYRVD PRAKLLTTFY FIIMIFLANN WVSYLVISIF GLAYVFATGL KARVFWDGVK PMIWMIVFTS LLQTFFMAG GKVYWHWWIF TLSSEGLING LYVFIRFAMI ILVSTVMTVT TKPLEIADAM EWMLTPLKLF KVNVGMISLV I SIALRFVP TLFDQTVKIM NAQRSRGADF NDGGLVKRAK SVVPMLVPLF IDSLEVALDL STAMESRGYK GSEGRTRYRI LE WSKVDLI PVAYCLLLTI LMITTRKH UniProtKB: Energy-coupling factor transporter transmembrane protein EcfT |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 5.4 mg/mL |
|---|---|
| Buffer | pH: 8 / Details: 20 mM Tris, pH 8.0, 150 mM NaCl |
| Grid | Model: Quantifoil R1.2/1.3 / Material: GOLD / Mesh: 300 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 45 sec. / Details: Edwards Scancoat 6, 5 mA |
| Vitrification | Cryogen name: ETHANE-PROPANE / Chamber humidity: 100 % / Chamber temperature: 288 K / Instrument: FEI VITROBOT MARK IV Details: 2.9 mM fluorinated Fos-choline 8 was added prior to sample application onto grids. Grids were blotted for 3.5-4 sec.. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TALOS ARCTICA |
|---|---|
| Specialist optics | Energy filter - Name: GIF Bioquantum / Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Digitization - Dimensions - Width: 3838 pixel / Digitization - Dimensions - Height: 3710 pixel / Digitization - Frames/image: 1-60 / Number grids imaged: 4 / Number real images: 12071 / Average exposure time: 9.0 sec. / Average electron dose: 50.1 e/Å2 Details: Dataset 1: 2200 movies Dataset 2: 4608 movie Dataset 3: 2101 movie Dataset 4: 3162 movie |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 100.0 µm / Calibrated defocus max: 1.8 µm / Calibrated defocus min: 0.8 µm / Calibrated magnification: 48924 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 1.8 µm / Nominal defocus min: 0.8 µm / Nominal magnification: 130000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Talos Arctica / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: RIGID BODY FIT |
|---|---|
| Output model |  PDB-8bmr: |
 Movie
Movie Controller
Controller









 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)