+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
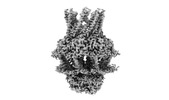
| Title | S. thermodepolymerans KpsMT(E151Q)-KpsE in complex with ATP | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | ABC transporter / Capsular polysaccharide / MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationlipopolysaccharide transport / ABC-type transporter activity / ATP-binding cassette (ABC) transporter complex / protein tyrosine kinase activity / ATP hydrolysis activity / ATP binding / plasma membrane Similarity search - Function | |||||||||
| Biological species |  Caldimonas thermodepolymerans (bacteria) Caldimonas thermodepolymerans (bacteria) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.1 Å | |||||||||
 Authors Authors | Kuklewicz J / Zimmer J | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nature / Year: 2024 Journal: Nature / Year: 2024Title: Molecular insights into capsular polysaccharide secretion. Authors: Jeremi Kuklewicz / Jochen Zimmer /  Abstract: Capsular polysaccharides (CPSs) fortify the cell boundaries of many commensal and pathogenic bacteria. Through the ABC-transporter-dependent biosynthesis pathway, CPSs are synthesized intracellularly ...Capsular polysaccharides (CPSs) fortify the cell boundaries of many commensal and pathogenic bacteria. Through the ABC-transporter-dependent biosynthesis pathway, CPSs are synthesized intracellularly on a lipid anchor and secreted across the cell envelope by the KpsMT ABC transporter associated with the KpsE and KpsD subunits. Here we use structural and functional studies to uncover crucial steps of CPS secretion in Gram-negative bacteria. We show that KpsMT has broad substrate specificity and is sufficient for the translocation of CPSs across the inner bacterial membrane, and we determine the cell surface organization and localization of CPSs using super-resolution fluorescence microscopy. Cryo-electron microscopy analyses of the KpsMT-KpsE complex in six different states reveal a KpsE-encaged ABC transporter, rigid-body conformational rearrangements of KpsMT during ATP hydrolysis and recognition of a glycolipid inside a membrane-exposed electropositive canyon. In vivo CPS secretion assays underscore the functional importance of canyon-lining basic residues. Combined, our analyses suggest a molecular model of CPS secretion by ABC transporters. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_41592.map.gz emd_41592.map.gz | 137.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-41592-v30.xml emd-41592-v30.xml emd-41592.xml emd-41592.xml | 15.7 KB 15.7 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_41592.png emd_41592.png | 44.7 KB | ||
| Filedesc metadata |  emd-41592.cif.gz emd-41592.cif.gz | 6.4 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-41592 http://ftp.pdbj.org/pub/emdb/structures/EMD-41592 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-41592 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-41592 | HTTPS FTP |
-Related structure data
| Related structure data |  8tshMC  8tsiC  8tslC  8tswC  8tt3C  8tunC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_41592.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_41592.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.08 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
- Sample components
Sample components
-Entire : ABC transporter KpsMT(E151Q) in complex with polysaccharide co-po...
| Entire | Name: ABC transporter KpsMT(E151Q) in complex with polysaccharide co-polymerase KpsE in ATP-bound state |
|---|---|
| Components |
|
-Supramolecule #1: ABC transporter KpsMT(E151Q) in complex with polysaccharide co-po...
| Supramolecule | Name: ABC transporter KpsMT(E151Q) in complex with polysaccharide co-polymerase KpsE in ATP-bound state type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#3 |
|---|---|
| Source (natural) | Organism:  Caldimonas thermodepolymerans (bacteria) Caldimonas thermodepolymerans (bacteria) |
-Macromolecule #1: ABC transporter ATP-binding protein
| Macromolecule | Name: ABC transporter ATP-binding protein / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Caldimonas thermodepolymerans (bacteria) Caldimonas thermodepolymerans (bacteria) |
| Molecular weight | Theoretical: 26.223744 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MIELRNLTKW YPTPHGRRYV FRNLNFRFPD DVSIGLIGRN GAGKSTLMRL LGGIEAPNEG EVVTDVSISW PVGLSGGFQG SLTARENVK FVCRIYGTSH EDMLRKVRFV EEFAEIGEHF DLPMKTYSSG MRSRVAFGLS MAFDFDYYLI DQAMAVGDAQ F RAKSRAVF ...String: MIELRNLTKW YPTPHGRRYV FRNLNFRFPD DVSIGLIGRN GAGKSTLMRL LGGIEAPNEG EVVTDVSISW PVGLSGGFQG SLTARENVK FVCRIYGTSH EDMLRKVRFV EEFAEIGEHF DLPMKTYSSG MRSRVAFGLS MAFDFDYYLI DQAMAVGDAQ F RAKSRAVF DSRVGQANMI LVSHNMNDIK EYCDVVVLVD QGQATLYEDV EAGIAAYQGS LKKAAAKPDY KDDDDK UniProtKB: ABC transporter ATP-binding protein |
-Macromolecule #2: Transport permease protein
| Macromolecule | Name: Transport permease protein / type: protein_or_peptide / ID: 2 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Caldimonas thermodepolymerans (bacteria) Caldimonas thermodepolymerans (bacteria) |
| Molecular weight | Theoretical: 30.800959 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MGKIHLAVSE RSPRVKRSPW QIQQAVLFAL FLRELKTRLG GRWLGVFWVL LEPVAHIAVM TTLFSLAHRA AMPSIEYPVF LITGLIPFF MFRGLVTRLM EAIDSNRGLF AYRQVKPIDT VIARAMLEIS LQSIVYLIAL GTLGWLGFHF LPVRALELAG V SAVLIMLG ...String: MGKIHLAVSE RSPRVKRSPW QIQQAVLFAL FLRELKTRLG GRWLGVFWVL LEPVAHIAVM TTLFSLAHRA AMPSIEYPVF LITGLIPFF MFRGLVTRLM EAIDSNRGLF AYRQVKPIDT VIARAMLEIS LQSIVYLIAL GTLGWLGFHF LPVRALELAG V SAVLIMLG ASLGLFFAVV TNEIPQARAI VRISLLPLYF VSGVIFPVHT IPPQYLPLLQ LNPVLHLIEL SRASFFPQYR VL QGINLAY PAGFALLSLF LALMLYRLRR HQLASVV UniProtKB: Transport permease protein |
-Macromolecule #3: Capsular biosynthesis protein
| Macromolecule | Name: Capsular biosynthesis protein / type: protein_or_peptide / ID: 3 / Number of copies: 8 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Caldimonas thermodepolymerans (bacteria) Caldimonas thermodepolymerans (bacteria) |
| Molecular weight | Theoretical: 44.057266 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MGKIHMKLVS RLTAKRLQWA LVYLPMLVAT VYFLVFSADR YVSESVITVR QTSSNAPTGG MSGAALLLAG LTPASREDTC YLQTYIHSM GLLQKLDQQL KLREHFGTPL RDPLFRLWGG TSQEWFLEYY RSRVEVLMDD ICGLLTVRVQ GFEPEFAQAL N RAILEESE ...String: MGKIHMKLVS RLTAKRLQWA LVYLPMLVAT VYFLVFSADR YVSESVITVR QTSSNAPTGG MSGAALLLAG LTPASREDTC YLQTYIHSM GLLQKLDQQL KLREHFGTPL RDPLFRLWGG TSQEWFLEYY RSRVEVLMDD ICGLLTVRVQ GFEPEFAQAL N RAILEESE RFVNELSHRM AREQGQFAEA ELERATARLQ EAKRQLIAFQ AKHKLLDPLA QAQATGTLTA ELQAALTRQE AE LRNALTY LNEDSYQVKA LRSQINALRQ QIDEERLRAT AGKNGDRINA VAAEFHDLQL QVGFAEDAYK LALAAVESAR IEA TRKLKS LVVVEPPVLP EIAEYPRRWY NLATLLVVCC LIYGVVSLVV ATIRDHQDGS GSGSHHHHHH HHHH UniProtKB: Capsular biosynthesis protein |
-Macromolecule #4: ADENOSINE-5'-TRIPHOSPHATE
| Macromolecule | Name: ADENOSINE-5'-TRIPHOSPHATE / type: ligand / ID: 4 / Number of copies: 2 / Formula: ATP |
|---|---|
| Molecular weight | Theoretical: 507.181 Da |
| Chemical component information |  ChemComp-ATP: |
-Macromolecule #5: MAGNESIUM ION
| Macromolecule | Name: MAGNESIUM ION / type: ligand / ID: 5 / Number of copies: 2 / Formula: MG |
|---|---|
| Molecular weight | Theoretical: 24.305 Da |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON III (4k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 1.8 µm / Nominal defocus min: 1.0 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller






















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)




















