+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | E. coli MlaC bound to MlaFEDB | |||||||||
 Map data Map data | E. coli MlaFEDB bound to MlaC, Map 1 | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | E. coli / bacteria / outer membrane / cryo-EM / lipid transport / mla / structural biology | |||||||||
| Biological species |  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 6.6 Å | |||||||||
 Authors Authors | MacRae MR / Coudray N / Ekiert D / Bhabha G | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: J Biol Chem / Year: 2023 Journal: J Biol Chem / Year: 2023Title: Protein-protein interactions in the Mla lipid transport system probed by computational structure prediction and deep mutational scanning. Authors: Mark R MacRae / Dhenesh Puvanendran / Max A B Haase / Nicolas Coudray / Ljuvica Kolich / Cherry Lam / Minkyung Baek / Gira Bhabha / Damian C Ekiert /  Abstract: The outer membrane (OM) of Gram-negative bacteria is an asymmetric bilayer that protects the cell from external stressors, such as antibiotics. The Mla transport system is implicated in the ...The outer membrane (OM) of Gram-negative bacteria is an asymmetric bilayer that protects the cell from external stressors, such as antibiotics. The Mla transport system is implicated in the Maintenance of OM Lipid Asymmetry by mediating retrograde phospholipid transport across the cell envelope. Mla uses a shuttle-like mechanism to move lipids between the MlaFEDB inner membrane complex and the MlaA-OmpF/C OM complex, via a periplasmic lipid-binding protein, MlaC. MlaC binds to MlaD and MlaA, but the underlying protein-protein interactions that facilitate lipid transfer are not well understood. Here, we take an unbiased deep mutational scanning approach to map the fitness landscape of MlaC from Escherichia coli, which provides insights into important functional sites. Combining this analysis with AlphaFold2 structure predictions and binding experiments, we map the MlaC-MlaA and MlaC-MlaD protein-protein interfaces. Our results suggest that the MlaD and MlaA binding surfaces on MlaC overlap to a large extent, leading to a model in which MlaC can only bind one of these proteins at a time. Low-resolution cryo-electron microscopy (cryo-EM) maps of MlaC bound to MlaFEDB suggest that at least two MlaC molecules can bind to MlaD at once, in a conformation consistent with AlphaFold2 predictions. These data lead us to a model for MlaC interaction with its binding partners and insights into lipid transfer steps that underlie phospholipid transport between the bacterial inner and OMs. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_40162.map.gz emd_40162.map.gz | 52 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-40162-v30.xml emd-40162-v30.xml emd-40162.xml emd-40162.xml | 28.6 KB 28.6 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_40162.png emd_40162.png | 38.6 KB | ||
| Masks |  emd_40162_msk_1.map emd_40162_msk_1.map | 107.2 MB |  Mask map Mask map | |
| Others |  emd_40162_additional_1.map.gz emd_40162_additional_1.map.gz emd_40162_additional_2.map.gz emd_40162_additional_2.map.gz emd_40162_additional_3.map.gz emd_40162_additional_3.map.gz emd_40162_half_map_1.map.gz emd_40162_half_map_1.map.gz emd_40162_half_map_2.map.gz emd_40162_half_map_2.map.gz | 53.2 MB 53 MB 52.1 MB 99.3 MB 99.3 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-40162 http://ftp.pdbj.org/pub/emdb/structures/EMD-40162 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-40162 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-40162 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_40162.map.gz / Format: CCP4 / Size: 107.2 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_40162.map.gz / Format: CCP4 / Size: 107.2 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | E. coli MlaFEDB bound to MlaC, Map 1 | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.096 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_40162_msk_1.map emd_40162_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
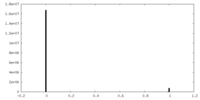
| Density Histograms |
-Additional map: E. coli MlaFEDB bound to MlaC, Map 2
| File | emd_40162_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | E. coli MlaFEDB bound to MlaC, Map 2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
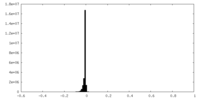
| Density Histograms |
-Additional map: E. coli MlaFEDB bound to MlaC, Map 3
| File | emd_40162_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | E. coli MlaFEDB bound to MlaC, Map 3 | ||||||||||||
| Projections & Slices |
| ||||||||||||
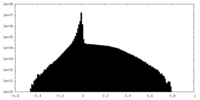
| Density Histograms |
-Additional map: E. coli MlaFEDB bound to MlaC, Map 4
| File | emd_40162_additional_3.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | E. coli MlaFEDB bound to MlaC, Map 4 | ||||||||||||
| Projections & Slices |
| ||||||||||||
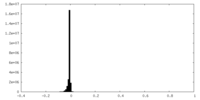
| Density Histograms |
-Half map: E. coli MlaFEDB bound to MlaC, half map A of Map 1
| File | emd_40162_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | E. coli MlaFEDB bound to MlaC, half map A of Map 1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: E. coli MlaFEDB bound to MlaC, half map B of Map 1
| File | emd_40162_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | E. coli MlaFEDB bound to MlaC, half map B of Map 1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : E. coli MlaC bound to MlaFEDB
| Entire | Name: E. coli MlaC bound to MlaFEDB |
|---|---|
| Components |
|
-Supramolecule #1: E. coli MlaC bound to MlaFEDB
| Supramolecule | Name: E. coli MlaC bound to MlaFEDB / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: MlaE
| Macromolecule | Name: MlaE / type: protein_or_peptide / ID: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Recombinant expression | Organism:  |
| Sequence | String: MLLNALASLG HKGIKTLRTF GRAGLMLFNA LVGKPEFRKH APLLVRQLYN VGVLSMLIIV VSGVFIGMVL GLQGYLVLTT YSAETSLGML VALSLLRELG PVVAALLFAG RAGSALTAEI GLMRATEQLS SMEMMAVDPL RRVISPRFWA GVISLPLLTV IFVAVGIWGG ...String: MLLNALASLG HKGIKTLRTF GRAGLMLFNA LVGKPEFRKH APLLVRQLYN VGVLSMLIIV VSGVFIGMVL GLQGYLVLTT YSAETSLGML VALSLLRELG PVVAALLFAG RAGSALTAEI GLMRATEQLS SMEMMAVDPL RRVISPRFWA GVISLPLLTV IFVAVGIWGG SLVGVSWKGI DSGFFWSAMQ NAVDWRMDLV NCLIKSVVFA ITVTWISLFN GYDAIPTSAG ISRATTRTVV HSSLAVLGLD FVLTALMFGN |
-Macromolecule #2: MlaF
| Macromolecule | Name: MlaF / type: protein_or_peptide / ID: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Recombinant expression | Organism:  |
| Sequence | String: MEQSVANLVD MRDVSFTRG N RCIFDNIS LT VPRGKIT AIM GPSGIG KTTL LRLIG GQIAP DHGE ILFDGE NIP AMSRSRL YT VRKRMSML F QSGALFTDM NVFDNVAYPL REHTQLPAP L LHSTVMMK LE AVGLRGA AKL MPSELS GGMA RRAAL ...String: MEQSVANLVD MRDVSFTRG N RCIFDNIS LT VPRGKIT AIM GPSGIG KTTL LRLIG GQIAP DHGE ILFDGE NIP AMSRSRL YT VRKRMSML F QSGALFTDM NVFDNVAYPL REHTQLPAP L LHSTVMMK LE AVGLRGA AKL MPSELS GGMA RRAAL ARAIA LEPD LIMFDE PFV GQDPITM GV LVKLISEL N SALGVTCVV VSHDVPEVLS IADHAWILA D KKIVAHGS AQ ALQANPD PRV RQFLDG IADG PVPFR YPAGD YHAD LLPGS |
-Macromolecule #3: MlaD
| Macromolecule | Name: MlaD / type: protein_or_peptide / ID: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Recombinant expression | Organism:  |
| Sequence | String: MHHHHHHQHQ HENLYFQGMQ TKKNEIWV GIFLLAALLA ALFVCLKAA N VTSIRTEP TY TLYATFD NIG GLKARS PVSI GGVVV GRVAD ITLD PKTYLP RVT LEIEQRY NH IPDTSSLS I RTSGLLGEQ YLALNVGFED PELGTAILK D GDTIQDTK SA ...String: MHHHHHHQHQ HENLYFQGMQ TKKNEIWV GIFLLAALLA ALFVCLKAA N VTSIRTEP TY TLYATFD NIG GLKARS PVSI GGVVV GRVAD ITLD PKTYLP RVT LEIEQRY NH IPDTSSLS I RTSGLLGEQ YLALNVGFED PELGTAILK D GDTIQDTK SA MVLEDLI GQF LYGSKG DDNK NSGDA PAAAP GNNE TTEPVG TTK |
-Macromolecule #4: MlaB
| Macromolecule | Name: MlaB / type: protein_or_peptide / ID: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Recombinant expression | Organism:  |
| Sequence | String: MSESLSWMQT GDTLALSGE L DQDVLLPL WE MREEAVK GIT CIDLSR VSRV DTGGL ALLLH LIDL AKKQGN NVT LQGVNDK VY TLAKLYNL P ADVLPR |
-Macromolecule #5: Foldon-MlaC
| Macromolecule | Name: Foldon-MlaC / type: protein_or_peptide / ID: 5 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Recombinant expression | Organism:  |
| Sequence | String: MHHHHHHPGS GYIPEAPRDG QAYVRKDGEW VLLSTFLSSG GGSGGGSGGG SSSGGGSGGG SGGGSSSGGG SGGGSGGGSA DQTNPYKLMD EAAQKTFDRL KNEQPQIRAN PDYLRTIVDQ ELLPYVQVKY AGALVLGQYY KSATPAQREA YFAAFREYLK QAYGQALAMY ...String: MHHHHHHPGS GYIPEAPRDG QAYVRKDGEW VLLSTFLSSG GGSGGGSGGG SSSGGGSGGG SGGGSSSGGG SGGGSGGGSA DQTNPYKLMD EAAQKTFDRL KNEQPQIRAN PDYLRTIVDQ ELLPYVQVKY AGALVLGQYY KSATPAQREA YFAAFREYLK QAYGQALAMY HGQTYQIAPE QPLGDKTIVP IRVTIIDPNG RPPVRLDFQW RKNSQTGNWQ AYDMIAEGVS MITTKQNEWG TLLRTKGIDG LTAQLKSISQ QKITLEEKK |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.2 mg/mL | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 8 Component:
Details: 10 mM Na2HPO4.7H2O, 137 mM NaCl, 2.7 mM KCl, 1.5 mM KH2PO4, 0.25 mM DDM | ||||||||||||||||||
| Grid | Model: Quantifoil R2/2 / Material: COPPER / Mesh: 300 / Support film - Material: CARBON / Support film - topology: CONTINUOUS / Support film - Film thickness: 2 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 5 sec. | ||||||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 293 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TECNAI ARCTICA |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 53.0 e/Å2 / Details: Pixel size = 0.5480 Angstrom |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.6 µm / Nominal defocus min: 1.1 µm / Nominal magnification: 36000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Talos Arctica / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: RIGID BODY FIT |
|---|
-Atomic model buiding 2
| Initial model | PDB ID: Chain - Source name: ModelArchive / Chain - Initial model type: in silico model / Details: computed with AlphaFold2 |
|---|
 Movie
Movie Controller
Controller





 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)