+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | EsN-dhsU36mm1 full map (map 1) | |||||||||
 Map data Map data | Raw map | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | promoter-bound / initiation / TRANSCRIPTION | |||||||||
| Function / homology |  Function and homology information Function and homology informationarginine metabolic process / DNA-binding transcription activator activity / RNA polymerase complex / submerged biofilm formation / cellular response to cell envelope stress / regulation of DNA-templated transcription initiation / sigma factor activity / bacterial-type flagellum assembly / bacterial-type RNA polymerase core enzyme binding / cytosolic DNA-directed RNA polymerase complex ...arginine metabolic process / DNA-binding transcription activator activity / RNA polymerase complex / submerged biofilm formation / cellular response to cell envelope stress / regulation of DNA-templated transcription initiation / sigma factor activity / bacterial-type flagellum assembly / bacterial-type RNA polymerase core enzyme binding / cytosolic DNA-directed RNA polymerase complex / bacterial-type flagellum-dependent cell motility / nitrate assimilation / nucleotidyltransferase activity / regulation of DNA-templated transcription elongation / transcription elongation factor complex / transcription antitermination / DNA-directed RNA polymerase complex / cell motility / DNA-templated transcription initiation / protein-DNA complex / ribonucleoside binding / DNA-directed RNA polymerase / DNA-directed RNA polymerase activity / response to heat / protein-containing complex assembly / intracellular iron ion homeostasis / protein dimerization activity / transcription cis-regulatory region binding / response to antibiotic / regulation of DNA-templated transcription / DNA-templated transcription / positive regulation of DNA-templated transcription / magnesium ion binding / DNA binding / zinc ion binding / membrane / cytoplasm / cytosol Similarity search - Function | |||||||||
| Biological species |    Aquifex aeolicus (bacteria) Aquifex aeolicus (bacteria) | |||||||||
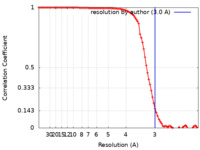
| Method | single particle reconstruction / cryo EM / Resolution: 3.0 Å | |||||||||
 Authors Authors | Mueller AU / Chen J / Darst SA | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Proc Natl Acad Sci U S A / Year: 2023 Journal: Proc Natl Acad Sci U S A / Year: 2023Title: A general mechanism for transcription bubble nucleation in bacteria. Authors: Andreas U Mueller / James Chen / Mengyu Wu / Courtney Chiu / B Tracy Nixon / Elizabeth A Campbell / Seth A Darst /  Abstract: Bacterial transcription initiation requires σ factors for nucleation of the transcription bubble. The canonical housekeeping σ factor, σ, nucleates DNA melting via recognition of conserved bases ...Bacterial transcription initiation requires σ factors for nucleation of the transcription bubble. The canonical housekeeping σ factor, σ, nucleates DNA melting via recognition of conserved bases of the promoter -10 motif, which are unstacked and captured in pockets of σ. By contrast, the mechanism of transcription bubble nucleation and formation during the unrelated σ-mediated transcription initiation is poorly understood. Herein, we combine structural and biochemical approaches to establish that σ, like σ, captures a flipped, unstacked base in a pocket formed between its N-terminal region I (RI) and extra-long helix features. Strikingly, RI inserts into the nascent bubble to stabilize the nucleated bubble prior to engagement of the obligate ATPase activator. Our data suggest a general paradigm of transcription initiation that requires σ factors to nucleate an early melted intermediate prior to productive RNA synthesis. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_28792.map.gz emd_28792.map.gz | 32.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-28792-v30.xml emd-28792-v30.xml emd-28792.xml emd-28792.xml | 31.1 KB 31.1 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_28792_fsc.xml emd_28792_fsc.xml | 8.9 KB | Display |  FSC data file FSC data file |
| Images |  emd_28792.png emd_28792.png | 108.3 KB | ||
| Masks |  emd_28792_msk_1.map emd_28792_msk_1.map emd_28792_msk_2.map emd_28792_msk_2.map | 64 MB 64 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-28792.cif.gz emd-28792.cif.gz | 8.2 KB | ||
| Others |  emd_28792_additional_1.map.gz emd_28792_additional_1.map.gz emd_28792_additional_2.map.gz emd_28792_additional_2.map.gz emd_28792_additional_3.map.gz emd_28792_additional_3.map.gz emd_28792_half_map_1.map.gz emd_28792_half_map_1.map.gz emd_28792_half_map_2.map.gz emd_28792_half_map_2.map.gz | 59.9 MB 51.9 MB 4.4 MB 59.4 MB 59.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-28792 http://ftp.pdbj.org/pub/emdb/structures/EMD-28792 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-28792 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-28792 | HTTPS FTP |
-Related structure data
| Related structure data |  8f1iC  8f1jC  8f1kC C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_28792.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_28792.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Raw map | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.083 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_28792_msk_1.map emd_28792_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Mask #2
| File |  emd_28792_msk_2.map emd_28792_msk_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: B-factor sharpened map (-131.3)
| File | emd_28792_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | B-factor sharpened map (-131.3) | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Locally filtered map (blocfilt)
| File | emd_28792_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Locally filtered map (blocfilt) | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Local resolution map (blocres)
| File | emd_28792_additional_3.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Local resolution map (blocres) | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map A
| File | emd_28792_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map A | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map B
| File | emd_28792_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map B | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : EsN-dhsU36mm1
| Entire | Name: EsN-dhsU36mm1 |
|---|---|
| Components |
|
-Supramolecule #1: EsN-dhsU36mm1
| Supramolecule | Name: EsN-dhsU36mm1 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#7 |
|---|
-Macromolecule #1: RNA polymerase alpha subunit
| Macromolecule | Name: RNA polymerase alpha subunit / type: protein_or_peptide / ID: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Recombinant expression | Organism:  |
| Sequence | String: MQGSVTEFLK PRLVDIEQVS STHAKVTLEP LERGFGHTLG NALRRILLSS MPGCAVTEVE IDGVLHEYST KEGVQEDILE ILLNLKGLA VRVQGKDEVI LTLNKSGIGP VTAADITHDG DVEIVKPQHV ICHLTDENAS ISMRIKVQRG RGYVPASTRI H SEEDERPI ...String: MQGSVTEFLK PRLVDIEQVS STHAKVTLEP LERGFGHTLG NALRRILLSS MPGCAVTEVE IDGVLHEYST KEGVQEDILE ILLNLKGLA VRVQGKDEVI LTLNKSGIGP VTAADITHDG DVEIVKPQHV ICHLTDENAS ISMRIKVQRG RGYVPASTRI H SEEDERPI GRLLVDACYS PVERIAYNVE AARVEQRTDL DKLVIEMETN GTIDPEEAIR RAATILAEQL EAFVDLRDVR QP EVKEEKP EFDPILLRPV DDLELTVRSA NCLKAEAIHY IGDLVQRTEV ELLKTPNLGK KSLTEIKDVL ASRGLSLGMR LEN WPPASI ADE UniProtKB: DNA-directed RNA polymerase subunit alpha |
-Macromolecule #2: RNA polymerase beta subunit
| Macromolecule | Name: RNA polymerase beta subunit / type: protein_or_peptide / ID: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Recombinant expression | Organism:  |
| Sequence | String: MVYSYTEKKR IRKDFGKRPQ VLDVPYLLSI QLDSFQKFIE QDPEGQYGLE AAFRSVFPIQ SYSGNSELQY VSYRLGEPVF DVQECQIRG VTYSAPLRVK LRLVIYEREA PEGTVKDIKE QEVYMGEIPL MTDNGTFVIN GTERVIVSQL HRSPGVFFDS D KGKTHSSG ...String: MVYSYTEKKR IRKDFGKRPQ VLDVPYLLSI QLDSFQKFIE QDPEGQYGLE AAFRSVFPIQ SYSGNSELQY VSYRLGEPVF DVQECQIRG VTYSAPLRVK LRLVIYEREA PEGTVKDIKE QEVYMGEIPL MTDNGTFVIN GTERVIVSQL HRSPGVFFDS D KGKTHSSG KVLYNARIIP YRGSWLDFEF DPKDNLFVRI DRRRKLPATI ILRALNYTTE QILDLFFEKV IFEIRDNKLQ ME LVPERLR GETASFDIEA NGKVYVEKGR RITARHIRQL EKDDVKLIEV PVEYIAGKVV AKDYIDESTG ELICAANMEL SLD LLAKLS QSGHKRIETL FTNDLDHGPY ISETLRVDPT NDRLSALVEI YRMMRPGEPP TREAAESLFE NLFFSEDRYD LSAV GRMKF NRSLLREEIE GSGILSKDDI IDVMKKLIDI RNGKGEVDDI DHLGNRRIRS VGEMAENQFR VGLVRVERAV KERLS LGDL DTLMPQDMIN AKPISAAVKE FFGSSQLSQF MDQNNPLSEI THKRRISALG PGGLTRERAG FEVRDVHPTH YGRVCP IET PEGPNIGLIN SLSVYAQTNE YGFLETPYRK VTDGVVTDEI HYLSAIEEGN YVIAQANSNL DEEGHFVEDL VTCRSKG ES SLFSRDQVDY MDVSTQQVVS VGASLIPFLE HDDANRALMG ANMQRQAVPT LRADKPLVGT GMERAVAVDS GVTAVAKR G GVVQYVDASR IVIKVNEDEM YPGEAGIDIY NLTKYTRSNQ NTCINQMPCV SLGEPVERGD VLADGPSTDL GELALGQNM RVAFMPWNGY NFEDSILVSE RVVQEDRFTT IHIQELACVS RDTKLGPEEI TADIPNVGEA ALSKLDESGI VYIGAEVTGG DILVGKVTP KGETQLTPEE KLLRAIFGEK ASDVKDSSLR VPNGVSGTVI DVQVFTRDGV EKDKRALEIE EMQLKQAKKD L SEELQILE AGLFSRIRAV LVAGGVEAEK LDKLPRDRWL ELGLTDEEKQ NQLEQLAEQY DELKHEFEKK LEAKRRKITQ GD DLAPGVL KIVKVYLAVK RRIQPGDKMA GRHGNKGVIS KINPIEDMPY DENGTPVDIV LNPLGVPSRM NIGQILETHL GMA AKGIGD KINAMLKQQQ EVAKLREFIQ RAYDLGADVR QKVDLSTFSD EEVMRLAENL RKGMPIATPV FDGAKEAEIK ELLK LGDLP TSGQIRLYDG RTGEQFERPV TVGYMYMLKL NHLVDDKMHA RSTGSYSLVT QQPLGGKAQF GGQRFGEMEV WALEA YGAA YTLQEMLTVK SDDVNGRTKM YKNIVDGNHQ MEPGMPESFN VLLKEIRSLG INIELEDE UniProtKB: DNA-directed RNA polymerase subunit beta |
-Macromolecule #3: RNA polymerase beta' subunit
| Macromolecule | Name: RNA polymerase beta' subunit / type: protein_or_peptide / ID: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Recombinant expression | Organism:  |
| Sequence | String: MKDLLKFLKA QTKTEEFDAI KIALASPDMI RSWSFGEVKK PETINYRTFK PERDGLFCAR IFGPVKDYEC LCGKYKRLKH RGVICEKCG VEVTQTKVRR ERMGHIELAS PTAHIWFLKS LPSRIGLLLD MPLRDIERVL YFESYVVIEG GMTNLERQQI L TEEQYLDA ...String: MKDLLKFLKA QTKTEEFDAI KIALASPDMI RSWSFGEVKK PETINYRTFK PERDGLFCAR IFGPVKDYEC LCGKYKRLKH RGVICEKCG VEVTQTKVRR ERMGHIELAS PTAHIWFLKS LPSRIGLLLD MPLRDIERVL YFESYVVIEG GMTNLERQQI L TEEQYLDA LEEFGDEFDA KMGAEAIQAL LKSMDLEQEC EQLREELNET NSETKRKKLT KRIKLLEAFV QSGNKPEWMI LT VLPVLPP DLRPLVPLDG GRFATSDLND LYRRVINRNN RLKRLLDLAA PDIIVRNEKR MLQEAVDALL DNGRRGRAIT GSN KRPLKS LADMIKGKQG RFRQNLLGKR VDYSGRSVIT VGPYLRLHQC GLPKKMALEL FKPFIYGKLE LRGLATTIKA AKKM VEREE AVVWDILDEV IREHPVLLNR APTLHRLGIQ AFEPVLIEGK AIQLHPLVCA AYNADFDGDQ MAVHVPLTLE AQLEA RALM MSTNNILSPA NGEPIIVPSQ DVVLGLYYMT RDCVNAKGEG MVLTGPKEAE RLYRSGLASL HARVKVRITE YEKDAN GEL VAKTSLKDTT VGRAILWMIV PKGLPYSIVN QALGKKAISK MLNTCYRILG LKPTVIFADQ IMYTGFAYAA RSGASVG ID DMVIPEKKHE IISEAEAEVA EIQEQFQSGL VTAGERYNKV IDIWAAANDR VSKAMMDNLQ TETVINRDGQ EEKQVSFN S IYMMADSGAR GSAAQIRQLA GMRGLMAKPD GSIIETPITA NFREGLNVLQ YFISTHGARK GLADTALKTA NSGYLTRRL VDVAQDLVVT EDDCGTHEGI MMTPVIEGGD VKEPLRDRVL GRVTAEDVLK PGTADILVPR NTLLHEQWCD LLEENSVDAV KVRSVVSCD TDFGVCAHCY GRDLARGHII NKGEAIGVIA AQSIGEPGTQ LTMRTFHIGG AASRAAAESS IQVKNKGSIK L SNVKSVVN SSGKLVITSR NTELKLIDEF GRTKESYKVP YGAVLAKGDG EQVAGGETVA NWDPHTMPVI TEVSGFVRFT DM IDGQTIT RQTDELTGLS SLVVLDSAER TAGGKDLRPA LKIVDAQGND VLIPGTDMPA QYFLPGKAIV QLEDGVQISS GDT LARIPQ ESGGTKDITG GLPRVADLFE ARRPKEPAIL AEISGIVSFG KETKGKRRLV ITPVDGSDPY EEMIPKWRQL NVFE GERVE RGDVISDGPE APHDILRLRG VHAVTRYIVN EVQDVYRLQG VKINDKHIEV IVRQMLRKAT IVNAGSSDFL EGEQV EYSR VKIANRELEA NGKVGATYSR DLLGITKASL ATESFISAAS FQETTRVLTE AAVAGKRDEL RGLKENVIVG RLIPAG TGY AYHQDRMRRR AAGEAPAAPQ VTAEDASASL AELLNAGLGG SDNELELEVL FQGPSSGHHH HHHHHHH UniProtKB: DNA-directed RNA polymerase subunit beta' |
-Macromolecule #4: RNA polymerase omega subunit
| Macromolecule | Name: RNA polymerase omega subunit / type: protein_or_peptide / ID: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Recombinant expression | Organism:  |
| Sequence | String: MARVTVQDAV EKIGNRFDLV LVAARRARQM QVGGKDPLVP EENDKTTVIA LREIEEGLIN NQILDVRERQ EQQEQEAAEL QAVTAIAEG RR UniProtKB: DNA-directed RNA polymerase subunit omega |
-Macromolecule #7: Sigma N / Sigma 54
| Macromolecule | Name: Sigma N / Sigma 54 / type: protein_or_peptide / ID: 7 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Recombinant expression | Organism:  |
| Sequence | String: SEFMKQGLQL RLSQQLAMTP QLQQAIRLLQ LSTLELQQEL QQALESNPLL EQIDTHEEID TRETQDSETL DTADALEQKE MPEELPLDA SWDTIYTAGT PSGTSGDYID DELPVYQGET TQTLQDYLMW QVELTPFSDT DRAIATSIVD AVDETGYLTV P LEDILESI ...String: SEFMKQGLQL RLSQQLAMTP QLQQAIRLLQ LSTLELQQEL QQALESNPLL EQIDTHEEID TRETQDSETL DTADALEQKE MPEELPLDA SWDTIYTAGT PSGTSGDYID DELPVYQGET TQTLQDYLMW QVELTPFSDT DRAIATSIVD AVDETGYLTV P LEDILESI GDEEIDIDEV EAVLKRIQRF DPVGVAAKDL RDCLLIQLSQ FDKTTPWLEE ARLIISDHLD LLANHDFRTL MR VTRLKED VLKEAVNLIQ SLDPRPGQSI QTGEPEYVIP DVLVRKHNGH WTVELNSDSI PRLQINQHYA SMCNNARNDG DSQ FIRSNL QDAKWLIKSL ESRNDTLLRV SRCIVEQQQA FFEQGEEYMK PMVLADIAQA VEMHESTISR VTTQKYLHSP RGIF ELKYF FSSHVNTEGG GEASSTAIRA LVKKLIAAEN PAKPLSDSKL TSLLSEQGIM VARRTVAKYR ESLSIPPSNQ RKQLV UniProtKB: RNA polymerase sigma-54 factor |
-Macromolecule #5: dhsU36mm1 top strand
| Macromolecule | Name: dhsU36mm1 top strand / type: dna / ID: 5 / Classification: DNA |
|---|---|
| Source (natural) | Organism:   Aquifex aeolicus (bacteria) Aquifex aeolicus (bacteria) |
| Sequence | String: (DC)(DC)(DA)(DG)(DA)(DA)(DA)(DT)(DT)(DG) (DG)(DC)(DA)(DC)(DG)(DA)(DA)(DA)(DA)(DT) (DT)(DG)(DC)(DC)(DT)(DT)(DA)(DA)(DA) (DT)(DA)(DC)(DA)(DA)(DC)(DG) |
-Macromolecule #6: dhsU36mm1 bottom strand
| Macromolecule | Name: dhsU36mm1 bottom strand / type: dna / ID: 6 / Classification: DNA |
|---|---|
| Source (natural) | Organism:   Aquifex aeolicus (bacteria) Aquifex aeolicus (bacteria) |
| Sequence | String: (DC)(DG)(DT)(DT)(DG)(DT)(DA)(DT)(DT)(DT) (DA)(DT)(DT)(DG)(DC)(DA)(DA)(DT)(DT)(DT) (DT)(DC)(DG)(DT)(DG)(DC)(DC)(DA)(DA) (DT)(DT)(DT)(DC)(DT)(DG)(DG) |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 Component:
| |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grid | Model: C-flat-1.2/1.3 / Material: GOLD / Mesh: 400 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 25 sec. / Pretreatment - Atmosphere: AIR Details: self-built glow discharge unit; chamber was evacuated using a vacuum pump prior to application of voltage (no pressure and/or voltage readings available) | |||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 295 K / Instrument: FEI VITROBOT MARK IV Details: Octyl beta-D-glucopyranoside (beta-OG) was added to the sample to 0.1%w/v final concentration (from 10x stock) just prior to plunge vitrification. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Energy filter - Name: GIF Bioquantum / Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Number real images: 12294 / Average exposure time: 2.0 sec. / Average electron dose: 51.0 e/Å2 Details: dose-fractionation with 0.05 seconds per frame (=40 frames) |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.5 µm / Nominal defocus min: 1.0 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller














 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)