[English] 日本語
 Yorodumi
Yorodumi- EMDB-27485: Cryo-electron tomogram of cryo-FIB milled E. coli with deletion o... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
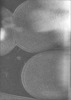
| Title | Cryo-electron tomogram of cryo-FIB milled E. coli with deletion of envC and nlpD. Low-passed. Septation stage. | ||||||||||||||||||
 Map data Map data | Cryo-electron tomogram of cryo-FIB milled E. coli with deletion of envC and nlpD. Low-passed. Septation stage. | ||||||||||||||||||
 Sample Sample |
| ||||||||||||||||||
 Keywords Keywords | Cell division / CELL CYCLE | ||||||||||||||||||
| Biological species |  | ||||||||||||||||||
| Method | electron tomography / cryo EM | ||||||||||||||||||
 Authors Authors | Navarro PP / Vettiger A / Ananda VY / Montero Llopis P / Allolio C / Bernhardt TG / Chao LH | ||||||||||||||||||
| Funding support |  Switzerland, European Union, Switzerland, European Union,  United States, 5 items United States, 5 items
| ||||||||||||||||||
 Citation Citation |  Journal: Nat Microbiol / Year: 2022 Journal: Nat Microbiol / Year: 2022Title: Cell wall synthesis and remodelling dynamics determine division site architecture and cell shape in Escherichia coli. Authors: Paula P Navarro / Andrea Vettiger / Virly Y Ananda / Paula Montero Llopis / Christoph Allolio / Thomas G Bernhardt / Luke H Chao /   Abstract: The bacterial division apparatus catalyses the synthesis and remodelling of septal peptidoglycan (sPG) to build the cell wall layer that fortifies the daughter cell poles. Understanding of this ...The bacterial division apparatus catalyses the synthesis and remodelling of septal peptidoglycan (sPG) to build the cell wall layer that fortifies the daughter cell poles. Understanding of this essential process has been limited by the lack of native three-dimensional views of developing septa. Here, we apply state-of-the-art cryogenic electron tomography (cryo-ET) and fluorescence microscopy to visualize the division site architecture and sPG biogenesis dynamics of the Gram-negative bacterium Escherichia coli. We identify a wedge-like sPG structure that fortifies the ingrowing septum. Experiments with strains defective in sPG biogenesis revealed that the septal architecture and mode of division can be modified to more closely resemble that of other Gram-negative (Caulobacter crescentus) or Gram-positive (Staphylococcus aureus) bacteria, suggesting that a conserved mechanism underlies the formation of different septal morphologies. Finally, analysis of mutants impaired in amidase activation (ΔenvC ΔnlpD) showed that cell wall remodelling affects the placement and stability of the cytokinetic ring. Taken together, our results support a model in which competition between the cell elongation and division machineries determines the shape of cell constrictions and the poles they form. They also highlight how the activity of the division system can be modulated to help generate the diverse array of shapes observed in the bacterial domain. #1:  Journal: Biorxiv / Year: 2021 Journal: Biorxiv / Year: 2021Title: Cell wall synthesis and remodeling dynamics determine bacterial division site architecture and cell shape Authors: Navarro PP / Vettiger A / Ananda VY / Llopis PM / Allolio C / Bernhardt TG / Chao LH | ||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_27485.map.gz emd_27485.map.gz | 161.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-27485-v30.xml emd-27485-v30.xml emd-27485.xml emd-27485.xml | 30.5 KB 30.5 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_27485.png emd_27485.png | 190.2 KB | ||
| Filedesc metadata |  emd-27485.cif.gz emd-27485.cif.gz | 4.5 KB | ||
| Others |  emd_27485_additional_1.map.gz emd_27485_additional_1.map.gz emd_27485_additional_2.map.gz emd_27485_additional_2.map.gz emd_27485_additional_3.map.gz emd_27485_additional_3.map.gz emd_27485_additional_4.map.gz emd_27485_additional_4.map.gz emd_27485_additional_5.map.gz emd_27485_additional_5.map.gz emd_27485_additional_6.map.gz emd_27485_additional_6.map.gz emd_27485_additional_7.map.gz emd_27485_additional_7.map.gz emd_27485_additional_8.map.gz emd_27485_additional_8.map.gz emd_27485_additional_9.map.gz emd_27485_additional_9.map.gz | 61.3 MB 119.4 MB 58.5 MB 35 MB 26.2 MB 184.4 MB 47.1 MB 50.2 MB 97.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-27485 http://ftp.pdbj.org/pub/emdb/structures/EMD-27485 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-27485 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-27485 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_27485.map.gz / Format: CCP4 / Size: 252.6 MB / Type: IMAGE STORED AS SIGNED INTEGER (2 BYTES) Download / File: emd_27485.map.gz / Format: CCP4 / Size: 252.6 MB / Type: IMAGE STORED AS SIGNED INTEGER (2 BYTES) | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Cryo-electron tomogram of cryo-FIB milled E. coli with deletion of envC and nlpD. Low-passed. Septation stage. | ||||||||||||||||||||||||||||||||
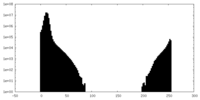
| Projections & slices | Image control
Images are generated by Spider. generated in cubic-lattice coordinate | ||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 20.52 Å | ||||||||||||||||||||||||||||||||
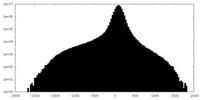
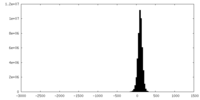
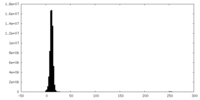
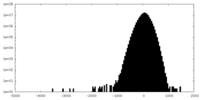
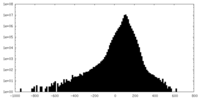
| Density |
| ||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: Cryo-electron tomogram of cryo-FIB milled E. coli with...
| File | emd_27485_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
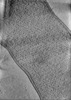
| Annotation | Cryo-electron tomogram of cryo-FIB milled E. coli with deletion of envC and nlpD. Septation stage. | ||||||||||||
| Projections & Slices |
| ||||||||||||
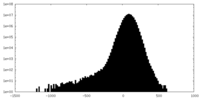
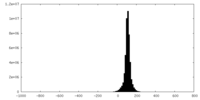
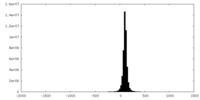
| Density Histograms |
-Additional map: Cryo-electron tomogram of cryo-FIB milled E. coli with...
| File | emd_27485_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Cryo-electron tomogram of cryo-FIB milled E. coli with deletion of envC and nlpD. Cytokinesis stage. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Cryo-electron tomogram of cryo-FIB milled E. coli with...
| File | emd_27485_additional_3.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Cryo-electron tomogram of cryo-FIB milled E. coli with deletion of envC and nlpD. Constriction stage. Double constriction. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Cryo-electron tomogram of cryo-FIB milled E. coli with...
| File | emd_27485_additional_4.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Cryo-electron tomogram of cryo-FIB milled E. coli with deletion of envC. Double constriction. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Cryo-electron tomogram of cryo-FIB milled E. coli with...
| File | emd_27485_additional_5.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Cryo-electron tomogram of cryo-FIB milled E. coli with deletion of envC. Cytokinesis stage. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Cryo-electron tomogram of cryo-FIB milled E. coli with...
| File | emd_27485_additional_6.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Cryo-electron tomogram of cryo-FIB milled E. coli with deletion of envC and nlpD. Septation stage. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Cryo-electron tomogram of cryo-FIB milled E. coli with...
| File | emd_27485_additional_7.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Cryo-electron tomogram of cryo-FIB milled E. coli with deletion of envC and nlpD. Low-passed. Constriction stage. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Cryo-electron tomogram of cryo-FIB milled E. coli with...
| File | emd_27485_additional_8.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Cryo-electron tomogram of cryo-FIB milled E. coli with deletion of envC and nlpD. Low-passed. Septation stage. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Cryo-electron tomogram of cryo-FIB milled E. coli with...
| File | emd_27485_additional_9.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Cryo-electron tomogram of cryo-FIB milled E. coli with deletion of envC and nlpD. Low-passed. Cytokinesis stage. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Dividing E. coli ftsN with deletion of SPOR domain
| Entire | Name: Dividing E. coli ftsN with deletion of SPOR domain |
|---|---|
| Components |
|
-Supramolecule #1: Dividing E. coli ftsN with deletion of SPOR domain
| Supramolecule | Name: Dividing E. coli ftsN with deletion of SPOR domain / type: cell / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | electron tomography |
| Aggregation state | cell |
- Sample preparation
Sample preparation
| Buffer | pH: 9 |
|---|---|
| Grid | Model: C-flat-2/2 / Material: COPPER / Support film - Material: CARBON / Support film - topology: HOLEY |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 294.15 K / Instrument: FEI VITROBOT MARK IV |
| Sectioning | Focused ion beam - Instrument: OTHER / Focused ion beam - Ion: OTHER / Focused ion beam - Voltage: 5 / Focused ion beam - Current: 0.5 / Focused ion beam - Duration: 600 / Focused ion beam - Temperature: 88 K / Focused ion beam - Initial thickness: 1000 / Focused ion beam - Final thickness: 150 Focused ion beam - Details: The value given for _em_focused_ion_beam.instrument is Aquilos 2. This is not in a list of allowed values {'OTHER', 'DB235'} so OTHER is written into the XML file. |
- Electron microscopy
Electron microscopy
| Microscope | TFS KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 1.5 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 5.5 µm / Nominal defocus min: 3.5 µm / Nominal magnification: 36000 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Final reconstruction | Software - Name:  IMOD / Number images used: 54 IMOD / Number images used: 54 |
|---|
 Movie
Movie Controller
Controller





 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)