[English] 日本語
 Yorodumi
Yorodumi- EMDB-26583: Cryo-EM structure of Antibody 12-16 in complex with prefusion SAR... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of Antibody 12-16 in complex with prefusion SARS-CoV-2 Spike glycoprotein | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Neutralizing Antibody / Viral Fusion Protein / SARS-CoV-2 / VIRAL PROTEIN-IMMUNE SYSTEM complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationsymbiont-mediated disruption of host tissue / Maturation of spike protein / Translation of Structural Proteins / Virion Assembly and Release / host cell surface / host extracellular space / symbiont-mediated-mediated suppression of host tetherin activity / Induction of Cell-Cell Fusion / structural constituent of virion / membrane fusion ...symbiont-mediated disruption of host tissue / Maturation of spike protein / Translation of Structural Proteins / Virion Assembly and Release / host cell surface / host extracellular space / symbiont-mediated-mediated suppression of host tetherin activity / Induction of Cell-Cell Fusion / structural constituent of virion / membrane fusion / entry receptor-mediated virion attachment to host cell / Attachment and Entry / host cell endoplasmic reticulum-Golgi intermediate compartment membrane / positive regulation of viral entry into host cell / receptor-mediated virion attachment to host cell / host cell surface receptor binding / symbiont-mediated suppression of host innate immune response / endocytosis involved in viral entry into host cell / receptor ligand activity / fusion of virus membrane with host plasma membrane / fusion of virus membrane with host endosome membrane / viral envelope / symbiont entry into host cell / virion attachment to host cell / host cell plasma membrane / SARS-CoV-2 activates/modulates innate and adaptive immune responses / virion membrane / identical protein binding / membrane / plasma membrane Similarity search - Function | |||||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.09 Å | |||||||||
 Authors Authors | Casner RG / Shapiro L | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: Immunity / Year: 2023 Journal: Immunity / Year: 2023Title: Antibodies targeting a quaternary site on SARS-CoV-2 spike glycoprotein prevent viral receptor engagement by conformational locking. Authors: Lihong Liu / Ryan G Casner / Yicheng Guo / Qian Wang / Sho Iketani / Jasper Fuk-Woo Chan / Jian Yu / Bernadeta Dadonaite / Manoj S Nair / Hiroshi Mohri / Eswar R Reddem / Shuofeng Yuan / ...Authors: Lihong Liu / Ryan G Casner / Yicheng Guo / Qian Wang / Sho Iketani / Jasper Fuk-Woo Chan / Jian Yu / Bernadeta Dadonaite / Manoj S Nair / Hiroshi Mohri / Eswar R Reddem / Shuofeng Yuan / Vincent Kwok-Man Poon / Chris Chung-Sing Chan / Kwok-Yung Yuen / Zizhang Sheng / Yaoxing Huang / Jesse D Bloom / Lawrence Shapiro / David D Ho /   Abstract: SARS-CoV-2 continues to evolve, with many variants evading clinically authorized antibodies. To isolate monoclonal antibodies (mAbs) with broadly neutralizing capacities against the virus, we ...SARS-CoV-2 continues to evolve, with many variants evading clinically authorized antibodies. To isolate monoclonal antibodies (mAbs) with broadly neutralizing capacities against the virus, we screened serum samples from convalescing COVID-19 patients. We isolated two mAbs, 12-16 and 12-19, which neutralized all SARS-CoV-2 variants tested, including the XBB subvariants, and prevented infection in hamsters challenged with Omicron BA.1 intranasally. Structurally, both antibodies targeted a conserved quaternary epitope located at the interface between the N-terminal domain and subdomain 1, uncovering a site of vulnerability on SARS-CoV-2 spike. These antibodies prevented viral receptor engagement by locking the receptor-binding domain (RBD) of spike in the down conformation, revealing a mechanism of virus neutralization for non-RBD antibodies. Deep mutational scanning showed that SARS-CoV-2 could mutate to escape 12-19, but such mutations are rarely found in circulating viruses. Antibodies 12-16 and 12-19 hold promise as prophylactic agents for immunocompromised persons who do not respond robustly to COVID-19 vaccines. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_26583.map.gz emd_26583.map.gz | 161.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-26583-v30.xml emd-26583-v30.xml emd-26583.xml emd-26583.xml | 18.2 KB 18.2 KB | Display Display |  EMDB header EMDB header |
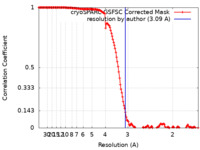
| FSC (resolution estimation) |  emd_26583_fsc.xml emd_26583_fsc.xml | 14.6 KB | Display |  FSC data file FSC data file |
| Images |  emd_26583.png emd_26583.png | 138.6 KB | ||
| Filedesc metadata |  emd-26583.cif.gz emd-26583.cif.gz | 6.5 KB | ||
| Others |  emd_26583_half_map_1.map.gz emd_26583_half_map_1.map.gz emd_26583_half_map_2.map.gz emd_26583_half_map_2.map.gz | 301.7 MB 301.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-26583 http://ftp.pdbj.org/pub/emdb/structures/EMD-26583 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-26583 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-26583 | HTTPS FTP |
-Related structure data
| Related structure data |  7uklMC  7ukmC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_26583.map.gz / Format: CCP4 / Size: 325 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_26583.map.gz / Format: CCP4 / Size: 325 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.83 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: half map B
| File | emd_26583_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map B | ||||||||||||
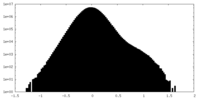
| Projections & Slices |
| ||||||||||||
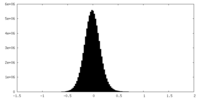
| Density Histograms |
-Half map: half map A
| File | emd_26583_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map A | ||||||||||||
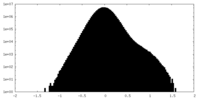
| Projections & Slices |
| ||||||||||||
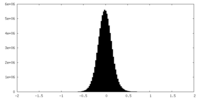
| Density Histograms |
- Sample components
Sample components
-Entire : Antibody 12-16 in complex with prefusion SARS-CoV-2 Spike glycoprotein
| Entire | Name: Antibody 12-16 in complex with prefusion SARS-CoV-2 Spike glycoprotein |
|---|---|
| Components |
|
-Supramolecule #1: Antibody 12-16 in complex with prefusion SARS-CoV-2 Spike glycoprotein
| Supramolecule | Name: Antibody 12-16 in complex with prefusion SARS-CoV-2 Spike glycoprotein type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#3 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: 12-16 Fab Light Chain
| Macromolecule | Name: 12-16 Fab Light Chain / type: protein_or_peptide / ID: 1 / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 11.669789 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: SYELTQPPSV SVSPGQTASI TCSGDKLGDK YACWYQQKPG QSPVLVIYQD NKRPSGIPER FSGSNSGNTA TLTISGTQAM DEADYYCQG WDRSTGYYVF GTGTKVTVL |
-Macromolecule #2: Spike glycoprotein
| Macromolecule | Name: Spike glycoprotein / type: protein_or_peptide / ID: 2 / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 141.205312 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MFVFLVLLPL VSSQCVNLTT RTQLPPAYTN SFTRGVYYPD KVFRSSVLHS TQDLFLPFFS NVTWFHAIHV SGTNGTKRFD NPVLPFNDG VYFASTEKSN IIRGWIFGTT LDSKTQSLLI VNNATNVVIK VCEFQFCNDP FLGVYYHKNN KSWMESEFRV Y SSANNCTF ...String: MFVFLVLLPL VSSQCVNLTT RTQLPPAYTN SFTRGVYYPD KVFRSSVLHS TQDLFLPFFS NVTWFHAIHV SGTNGTKRFD NPVLPFNDG VYFASTEKSN IIRGWIFGTT LDSKTQSLLI VNNATNVVIK VCEFQFCNDP FLGVYYHKNN KSWMESEFRV Y SSANNCTF EYVSQPFLMD LEGKQGNFKN LREFVFKNID GYFKIYSKHT PINLVRDLPQ GFSALEPLVD LPIGINITRF QT LLALHRS YLTPGDSSSG WTAGAAAYYV GYLQPRTFLL KYNENGTITD AVDCALDPLS ETKCTLKSFT VEKGIYQTSN FRV QPTESI VRFPNITNLC PFGEVFNATR FASVYAWNRK RISNCVADYS VLYNSASFST FKCYGVSPTK LNDLCFTNVY ADSF VIRGD EVRQIAPGQT GKIADYNYKL PDDFTGCVIA WNSNNLDSKV GGNYNYLYRL FRKSNLKPFE RDISTEIYQA GSTPC NGVE GFNCYFPLQS YGFQPTNGVG YQPYRVVVLS FELLHAPATV CGPKKSTNLV KNKCVNFNFN GLTGTGVLTE SNKKFL PFQ QFGRDIADTT DAVRDPQTLE ILDITPCSFG GVSVITPGTN TSNQVAVLYQ GVNCTEVPVA IHADQLTPTW RVYSTGS NV FQTRAGCLIG AEHVNNSYEC DIPIGAGICA SYQTQTNSPR RARSVASQSI IAYTMSLGAE NSVAYSNNSI AIPTNFTI S VTTEILPVSM TKTSVDCTMY ICGDSTECSN LLLQYGSFCT QLNRALTGIA VEQDKNTQEV FAQVKQIYKT PPIKDFGGF NFSQILPDPS KPSKRSFIED LLFNKVTLAD AGFIKQYGDC LGDIAARDLI CAQKFNGLTV LPPLLTDEMI AQYTSALLAG TITSGWTFG AGAALQIPFA MQMAYRFNGI GVTQNVLYEN QKLIANQFNS AIGKIQDSLS STASALGKLQ DVVNQNAQAL N TLVKQLSS NFGAISSVLN DILSRLDPPE AEVQIDRLIT GRLQSLQTYV TQQLIRAAEI RASANLAATK MSECVLGQSK RV DFCGKGY HLMSFPQSAP HGVVFLHVTY VPAQEKNFTT APAICHDGKA HFPREGVFVS NGTHWFVTQR NFYEPQIITT DNT FVSGNC DVVIGIVNNT VYDPLQPELD SFKEELDKYF KNHTSPDVDL GDISGINASV VNIQKEIDRL NEVAKNLNES LIDL QELGK YEQYIKWPWY IWLGFIAGLI AIVMVTIMLC CMTSCCSCLK GCCSCGSCCK FDEDDSEPVL KGVKLHYT UniProtKB: Spike glycoprotein |
-Macromolecule #3: 12-16 Fab Heavy Chain
| Macromolecule | Name: 12-16 Fab Heavy Chain / type: protein_or_peptide / ID: 3 / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 14.604067 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: QVQLVESGGG VVQPGRSLRL SCAASGFTFS NYGMHWVRQA PGKGLEWVAI ISFDGSNRYY ADSVKGRFTI SRDNSKNTLY LQMDSLRAE DTAVYYCAKD LSFYYDISTG YYPQSYNSGT DVWGQGTTVT VSS |
-Macromolecule #4: 2-acetamido-2-deoxy-beta-D-glucopyranose
| Macromolecule | Name: 2-acetamido-2-deoxy-beta-D-glucopyranose / type: ligand / ID: 4 / Number of copies: 48 / Formula: NAG |
|---|---|
| Molecular weight | Theoretical: 221.208 Da |
| Chemical component information |  ChemComp-NAG: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 1 mg/mL |
|---|---|
| Buffer | pH: 5.5 |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 58.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: -2.0 µm / Nominal defocus min: -0.8 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller








 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)