+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of human Kv1.3 with Fab-ShK fusion | |||||||||
 Map data Map data | Human Kv1.3 with Fab-ShK | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | ion channel / IMMUNE SYSTEM | |||||||||
| Function / homology |  Function and homology information Function and homology informationvoltage-gated monoatomic ion channel activity / delayed rectifier potassium channel activity / Voltage gated Potassium channels / action potential / voltage-gated potassium channel activity / voltage-gated potassium channel complex / potassium ion transmembrane transport / bioluminescence / generation of precursor metabolites and energy / potassium ion transport ...voltage-gated monoatomic ion channel activity / delayed rectifier potassium channel activity / Voltage gated Potassium channels / action potential / voltage-gated potassium channel activity / voltage-gated potassium channel complex / potassium ion transmembrane transport / bioluminescence / generation of precursor metabolites and energy / potassium ion transport / protein homooligomerization / axon / perinuclear region of cytoplasm / membrane / plasma membrane Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) /  | |||||||||
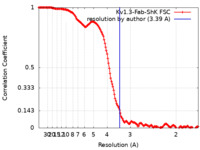
| Method | single particle reconstruction / cryo EM / Resolution: 3.39 Å | |||||||||
 Authors Authors | Meyerson JR / Selvakumar P | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2022 Journal: Nat Commun / Year: 2022Title: Structures of the T cell potassium channel Kv1.3 with immunoglobulin modulators. Authors: Purushotham Selvakumar / Ana I Fernández-Mariño / Nandish Khanra / Changhao He / Alice J Paquette / Bing Wang / Ruiqi Huang / Vaughn V Smider / William J Rice / Kenton J Swartz / Joel R Meyerson /  Abstract: The Kv1.3 potassium channel is expressed abundantly on activated T cells and mediates the cellular immune response. This role has made the channel a target for therapeutic immunomodulation to block ...The Kv1.3 potassium channel is expressed abundantly on activated T cells and mediates the cellular immune response. This role has made the channel a target for therapeutic immunomodulation to block its activity and suppress T cell activation. Here, we report structures of human Kv1.3 alone, with a nanobody inhibitor, and with an antibody-toxin fusion blocker. Rather than block the channel directly, four copies of the nanobody bind the tetramer's voltage sensing domains and the pore domain to induce an inactive pore conformation. In contrast, the antibody-toxin fusion docks its toxin domain at the extracellular mouth of the channel to insert a critical lysine into the pore. The lysine stabilizes an active conformation of the pore yet blocks ion permeation. This study visualizes Kv1.3 pore dynamics, defines two distinct mechanisms to suppress Kv1.3 channel activity with exogenous inhibitors, and provides a framework to aid development of emerging T cell immunotherapies. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_25414.map.gz emd_25414.map.gz | 307.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-25414-v30.xml emd-25414-v30.xml emd-25414.xml emd-25414.xml | 12.3 KB 12.3 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_25414_fsc.xml emd_25414_fsc.xml | 15.8 KB | Display |  FSC data file FSC data file |
| Images |  emd_25414.png emd_25414.png | 94.5 KB | ||
| Filedesc metadata |  emd-25414.cif.gz emd-25414.cif.gz | 6 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-25414 http://ftp.pdbj.org/pub/emdb/structures/EMD-25414 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-25414 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-25414 | HTTPS FTP |
-Related structure data
| Related structure data |  7ssvMC  7ssxC  7ssyC  7sszC  8dflC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
| EM raw data |  EMPIAR-11081 (Title: Human Kv1.3 with a Fab-ShK fusion / Data size: 2.0 TB EMPIAR-11081 (Title: Human Kv1.3 with a Fab-ShK fusion / Data size: 2.0 TBData #1: Human Kv1.3 ShK-Fab dataset [micrographs - multiframe]) |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_25414.map.gz / Format: CCP4 / Size: 325 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_25414.map.gz / Format: CCP4 / Size: 325 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Human Kv1.3 with Fab-ShK | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.852 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
- Sample components
Sample components
-Entire : Kv1.3 with Fab-ShK
| Entire | Name: Kv1.3 with Fab-ShK |
|---|---|
| Components |
|
-Supramolecule #1: Kv1.3 with Fab-ShK
| Supramolecule | Name: Kv1.3 with Fab-ShK / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#3 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Potassium voltage-gated channel subfamily A member 3,Green fluore...
| Macromolecule | Name: Potassium voltage-gated channel subfamily A member 3,Green fluorescent protein fusion type: protein_or_peptide / ID: 1 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 95.0185 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MDERLSLLRS PPPPSARHRA HPPQRPASSG GAHTLVNHGY AEPAAGRELP PDMTVVPGDH LLEPEVADGG GAPPQGGCGG GGCDRYEPL PPSLPAAGEQ DCCGERVVIN ISGLRFETQL KTLCQFPETL LGDPKRRMRY FDPLRNEYFF DRNRPSFDAI L YYYQSGGR ...String: MDERLSLLRS PPPPSARHRA HPPQRPASSG GAHTLVNHGY AEPAAGRELP PDMTVVPGDH LLEPEVADGG GAPPQGGCGG GGCDRYEPL PPSLPAAGEQ DCCGERVVIN ISGLRFETQL KTLCQFPETL LGDPKRRMRY FDPLRNEYFF DRNRPSFDAI L YYYQSGGR IRRPVNVPID IFSEEIRFYQ LGEEAMEKFR EDEGFLREEE RPLPRRDFQR QVWLLFEYPE SSGPARGIAI VS VLVILIS IVIFCLETLP EFRDEKDYPA STSQDSFEAA GNSTSGSRAG ASSFSDPFFV VETLCIIWFS FELLVRFFAC PSK ATFSRN IMNLIDIVAI IPYFITLGTE LAERQGNGQQ AMSLAILRVI RLVRVFRIFK LSRHSKGLQI LGQTLKASMR ELGL LIFFL FIGVILFSSA VYFAEADDPT SGFSSIPDAF WWAVVTMTTV GYGDMHPVTI GGKIVGSLCA IAGVLTIALP VPVIV SNFN YFYHRETEGE EQSQYMHVGS CQHLSSSAEE LRKARSNSTL SKSEYMVIEE GGMNHSAFPQ TPFKTGNSTA TCTTNN NPN SCVNIKKIFT DVSLEVLFQG PAAAMVSKGE ELFTGVVPIL VELDGDVNGH KFSVSGEGEG DATYGKLTLK LICTTGK LP VPWPTLVTTL GYGLQCFARY PDHMKQHDFF KSAMPEGYVQ ERTIFFKDDG NYKTRAEVKF EGDTLVNRIE LKGIDFKE D GNILGHKLEY NYNSHNVYIT ADKQKNGIKA NFKIRHNIED GGVQLADHYQ QNTPIGDGPV LLPDNHYLSY QSKLSKDPN EKRDHMVLLE FVTAAGITLG MDELYKSAWS HPQFEKGGGS GGGSGGGSWS HPQFEK UniProtKB: Potassium voltage-gated channel subfamily A member 3, Green fluorescent protein |
-Macromolecule #2: Fab-ShK fusion, heavy chain
| Macromolecule | Name: Fab-ShK fusion, heavy chain / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism: unidentified (others) |
| Molecular weight | Theoretical: 29.417229 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: QVQLREWGAG LLKPSETLSL TCAVYGGSFS DKYWSWIRQP PGKGLEWIGS INHSGSTNYN PSLKSRVTIS VDTSKNQFSL KLSSVTAAD TAVYYCTSVH QETKKYQSRS CIDTIPKSRC TAFQCKHSMK YRLSFCRKTC GTCSYTYNYE WHVDVWGQGL L VTVSSAST ...String: QVQLREWGAG LLKPSETLSL TCAVYGGSFS DKYWSWIRQP PGKGLEWIGS INHSGSTNYN PSLKSRVTIS VDTSKNQFSL KLSSVTAAD TAVYYCTSVH QETKKYQSRS CIDTIPKSRC TAFQCKHSMK YRLSFCRKTC GTCSYTYNYE WHVDVWGQGL L VTVSSAST KGPSVFPLAP SSKSTSGGTA ALGCLVKDYF PEPVTVSWNS GALTSGVHTF PAVLQSSGLY SLSSVVTVPS SS LGTQTYI CNVNHKPSNT KVDKKVEPKS CDK |
-Macromolecule #3: Fab-ShK fusion, light chain
| Macromolecule | Name: Fab-ShK fusion, light chain / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism: unidentified (others) |
| Molecular weight | Theoretical: 22.524752 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: QAVLNQPSSV SGSLGQKVTI SCSGSSSNIG NNYVSWYQQL PGTAPKLLIY GDTKRPSGIP DRFSGSKSGT SATLGITGLQ TGDEADYYC ASAEDSSSNA VFGSGTTLTV LGQPKAAPSV TLFPPSSEEL QANKATLVCL ISDFYPGAVT VAWKADSSPV K AGVETTTP ...String: QAVLNQPSSV SGSLGQKVTI SCSGSSSNIG NNYVSWYQQL PGTAPKLLIY GDTKRPSGIP DRFSGSKSGT SATLGITGLQ TGDEADYYC ASAEDSSSNA VFGSGTTLTV LGQPKAAPSV TLFPPSSEEL QANKATLVCL ISDFYPGAVT VAWKADSSPV K AGVETTTP SKQSNNKYAA SSYLSLTPEQ WKSHRSYSCQ VTHEGSTVEK TVAPTECS |
-Macromolecule #4: POTASSIUM ION
| Macromolecule | Name: POTASSIUM ION / type: ligand / ID: 4 / Number of copies: 3 / Formula: K |
|---|---|
| Molecular weight | Theoretical: 39.098 Da |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 54.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: OTHER / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller














 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)