[English] 日本語
 Yorodumi
Yorodumi- EMDB-25370: Chlorella virus Hyaluronan Synthase in the GlcNAc-primed, channel... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Chlorella virus Hyaluronan Synthase in the GlcNAc-primed, channel-open state | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | glycosyltransferase / hyaluronan / MEMBRANE PROTEIN | |||||||||
| Function / homology | hyaluronan synthase activity / Glycosyltransferase like family 2 / hyaluronan biosynthetic process / Nucleotide-diphospho-sugar transferases / plasma membrane / Hyaluronan synthase Function and homology information Function and homology information | |||||||||
| Biological species |  Paramecium bursaria Chlorella virus CZ-2 / Paramecium bursaria Chlorella virus CZ-2 /  | |||||||||
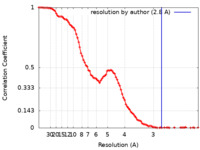
| Method | single particle reconstruction / cryo EM / Resolution: 2.8 Å | |||||||||
 Authors Authors | Maloney FP / Kuklewicz J | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nature / Year: 2022 Journal: Nature / Year: 2022Title: Structure, substrate recognition and initiation of hyaluronan synthase. Authors: Finn P Maloney / Jeremi Kuklewicz / Robin A Corey / Yunchen Bi / Ruoya Ho / Lukasz Mateusiak / Els Pardon / Jan Steyaert / Phillip J Stansfeld / Jochen Zimmer /     Abstract: Hyaluronan is an acidic heteropolysaccharide comprising alternating N-acetylglucosamine and glucuronic acid sugars that is ubiquitously expressed in the vertebrate extracellular matrix. The high- ...Hyaluronan is an acidic heteropolysaccharide comprising alternating N-acetylglucosamine and glucuronic acid sugars that is ubiquitously expressed in the vertebrate extracellular matrix. The high-molecular-mass polymer modulates essential physiological processes in health and disease, including cell differentiation, tissue homeostasis and angiogenesis. Hyaluronan is synthesized by a membrane-embedded processive glycosyltransferase, hyaluronan synthase (HAS), which catalyses the synthesis and membrane translocation of hyaluronan from uridine diphosphate-activated precursors. Here we describe five cryo-electron microscopy structures of a viral HAS homologue at different states during substrate binding and initiation of polymer synthesis. Combined with biochemical analyses and molecular dynamics simulations, our data reveal how HAS selects its substrates, hydrolyses the first substrate to prime the synthesis reaction, opens a hyaluronan-conducting transmembrane channel, ensures alternating substrate polymerization and coordinates hyaluronan inside its transmembrane pore. Our research suggests a detailed model for the formation of an acidic extracellular heteropolysaccharide and provides insights into the biosynthesis of one of the most abundant and essential glycosaminoglycans in the human body. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_25370.map.gz emd_25370.map.gz | 54.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-25370-v30.xml emd-25370-v30.xml emd-25370.xml emd-25370.xml | 17.5 KB 17.5 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_25370_fsc.xml emd_25370_fsc.xml | 11.7 KB | Display |  FSC data file FSC data file |
| Images |  emd_25370.png emd_25370.png | 67.6 KB | ||
| Filedesc metadata |  emd-25370.cif.gz emd-25370.cif.gz | 6.8 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-25370 http://ftp.pdbj.org/pub/emdb/structures/EMD-25370 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-25370 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-25370 | HTTPS FTP |
-Related structure data
| Related structure data |  7spaMC  7sp6C  7sp7C  7sp8C  7sp9C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
| EM raw data |  EMPIAR-11031 (Title: Cryo electron microscopy of inactive hyaluronan synthase with UDP-GlcNAc EMPIAR-11031 (Title: Cryo electron microscopy of inactive hyaluronan synthase with UDP-GlcNAcData size: 2.3 TB Data #1: Unaligned movies of Chlorella virus hyaluronan synthase [micrographs - multiframe]) |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_25370.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_25370.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.08 Å | ||||||||||||||||||||||||||||||||||||
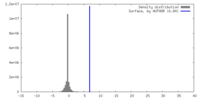
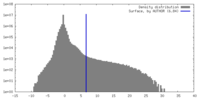
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
- Sample components
Sample components
-Entire : Hyaluronan synthase in nanodiscs in complex with two camelid nano...
| Entire | Name: Hyaluronan synthase in nanodiscs in complex with two camelid nanobodies. |
|---|---|
| Components |
|
-Supramolecule #1: Hyaluronan synthase in nanodiscs in complex with two camelid nano...
| Supramolecule | Name: Hyaluronan synthase in nanodiscs in complex with two camelid nanobodies. type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#3 |
|---|---|
| Source (natural) | Organism:  Paramecium bursaria Chlorella virus CZ-2 Paramecium bursaria Chlorella virus CZ-2 |
| Molecular weight | Theoretical: 95.8 KDa |
-Macromolecule #1: Nanobody 872
| Macromolecule | Name: Nanobody 872 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 14.783248 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: QVQLVESGGG LVQAGGSLKV SCAASGRAFK TYRMAWFRQA PGKEREFVSG ISALETTYYA DSVKGRFTIS RDNTKNTVSL QMDSLKPED TAVYYCAARR YGGTDYTTTG SYDYWGQGTQ VTVSSHHHHH HEPEA |
-Macromolecule #2: Nanobody 881
| Macromolecule | Name: Nanobody 881 / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 15.265755 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: QVQLVESGGG LVQAGGSLRL ACAASGRIFS SDTLAWFRRA PGKEREFVAA SRWSGGGTDY ADSVKGRFTF SRDNTRNTMC LEMNSLKPE DTAVYYCALR TARDSYYYTR NPTGYDYWGQ GTQVTVSSHH HHHHEPEA |
-Macromolecule #3: Hyaluronan synthase
| Macromolecule | Name: Hyaluronan synthase / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Paramecium bursaria Chlorella virus CZ-2 Paramecium bursaria Chlorella virus CZ-2 |
| Molecular weight | Theoretical: 65.336484 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MGTSWRTIVS ANLFAVGGAL LMLAPAIVGY VFQWNIGVSA VWGISVYGVF VLGFYIAQIV FSEFNRMRLS DWISLRPDNW NATRVAVII AGYREDPFMF KKCLESVRDS EYGNVARLIC VIDGDEEEDL KMAEIYKQVY NDNVKKPGVV LCESENKNGS T IDSDVSKN ...String: MGTSWRTIVS ANLFAVGGAL LMLAPAIVGY VFQWNIGVSA VWGISVYGVF VLGFYIAQIV FSEFNRMRLS DWISLRPDNW NATRVAVII AGYREDPFMF KKCLESVRDS EYGNVARLIC VIDGDEEEDL KMAEIYKQVY NDNVKKPGVV LCESENKNGS T IDSDVSKN ICILQPHRGK RESLYTGFQL ASMDPSVHAV VLIDSDTVLE KNAILEVVYP LSCDPNIKAV AGECKIWNTD TI LSMLVSW RYFSAFNVER GAQSLWKTVQ CVGGPLGAYT IDIINEIKDP WITQTFLGNK CTYGDNRRLT NEVLMRGKKI VYT PFAVGW SDSPTNVMRY IVQQTRWSKS WCREIWYTLG SAWKHGFSGI YLAFECMYQI MYFFLVMYLF SYIAIKADIR AQTA TVLVS TLVTIIKSSY LALRAKNLKA FYFVLYTYVY FFCMIPARIT AMFTMFDIAW GTRGGNAKMT IGARVWLWAK QFLIT YMWW AGVLAAGVYS IVDNWYFDWA DIQYRFALVG ICSYLVFVSI VLVIYLIGKI TTWNYTPLQK ELIEERYLHN ASENAP EVL EHHHHHH UniProtKB: Hyaluronan synthase |
-Macromolecule #4: 2-acetamido-2-deoxy-beta-D-glucopyranose
| Macromolecule | Name: 2-acetamido-2-deoxy-beta-D-glucopyranose / type: ligand / ID: 4 / Number of copies: 1 / Formula: NAG |
|---|---|
| Molecular weight | Theoretical: 221.208 Da |
| Chemical component information |  ChemComp-NAG: |
-Macromolecule #5: CHOLESTEROL HEMISUCCINATE
| Macromolecule | Name: CHOLESTEROL HEMISUCCINATE / type: ligand / ID: 5 / Number of copies: 1 / Formula: Y01 |
|---|---|
| Molecular weight | Theoretical: 486.726 Da |
| Chemical component information |  ChemComp-Y01: |
-Macromolecule #6: 1,2-Distearoyl-sn-glycerophosphoethanolamine
| Macromolecule | Name: 1,2-Distearoyl-sn-glycerophosphoethanolamine / type: ligand / ID: 6 / Number of copies: 1 / Formula: 3PE |
|---|---|
| Molecular weight | Theoretical: 748.065 Da |
| Chemical component information |  ChemComp-3PE: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 Component:
| ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Mesh: 300 / Support film - Material: CARBON / Support film - topology: HOLEY Details: Sample incubated on grid 30s before blotting. Blot 4 seconds with force 4. | ||||||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277.15 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Energy filter - Name: GIF Bioquantum / Energy filter - Slit width: 10 eV |
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Number grids imaged: 1 / Number real images: 8218 / Average electron dose: 51.0 e/Å2 / Details: 40-frame movies were collected |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.0 µm / Nominal defocus min: 1.0 µm / Nominal magnification: 81000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller









 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)