[English] 日本語
 Yorodumi
Yorodumi- EMDB-25076: LPHN3 (ADGRL3) 7TM domain bound to tethered agonist in complex wi... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | LPHN3 (ADGRL3) 7TM domain bound to tethered agonist in complex with G protein heterotrimer | |||||||||
 Map data Map data | Cryo-EM composite map for LPHN3 - miniG13 heterotrimer | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Adhesion GPCR / LPHN3 / Latrophilin / ADGRL3 / tethered agonist / stalk / stachel / miniG13 / G13 heterotrimer / G protein / cryoEM / membrane protein | |||||||||
| Function / homology |  Function and homology information Function and homology informationOlfactory Signaling Pathway / Activation of the phototransduction cascade / G beta:gamma signalling through PLC beta / Presynaptic function of Kainate receptors / Thromboxane signalling through TP receptor / G protein-coupled acetylcholine receptor signaling pathway / Activation of G protein gated Potassium channels / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / G-protein activation / G beta:gamma signalling through CDC42 ...Olfactory Signaling Pathway / Activation of the phototransduction cascade / G beta:gamma signalling through PLC beta / Presynaptic function of Kainate receptors / Thromboxane signalling through TP receptor / G protein-coupled acetylcholine receptor signaling pathway / Activation of G protein gated Potassium channels / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / G-protein activation / G beta:gamma signalling through CDC42 / Prostacyclin signalling through prostacyclin receptor / Glucagon signaling in metabolic regulation / G beta:gamma signalling through BTK / Synthesis, secretion, and inactivation of Glucagon-like Peptide-1 (GLP-1) / ADP signalling through P2Y purinoceptor 12 / photoreceptor disc membrane / Glucagon-type ligand receptors / Sensory perception of sweet, bitter, and umami (glutamate) taste / Adrenaline,noradrenaline inhibits insulin secretion / Vasopressin regulates renal water homeostasis via Aquaporins / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / G alpha (z) signalling events / ADP signalling through P2Y purinoceptor 1 / ADORA2B mediated anti-inflammatory cytokines production / cellular response to catecholamine stimulus / G beta:gamma signalling through PI3Kgamma / adenylate cyclase-activating dopamine receptor signaling pathway / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / GPER1 signaling / G-protein beta-subunit binding / cellular response to prostaglandin E stimulus / heterotrimeric G-protein complex / G alpha (12/13) signalling events / Inactivation, recovery and regulation of the phototransduction cascade / extracellular vesicle / sensory perception of taste / Thrombin signalling through proteinase activated receptors (PARs) / signaling receptor complex adaptor activity / retina development in camera-type eye / GTPase binding / Ca2+ pathway / fibroblast proliferation / High laminar flow shear stress activates signaling by PIEZO1 and PECAM1:CDH5:KDR in endothelial cells / G alpha (i) signalling events / G alpha (s) signalling events / phospholipase C-activating G protein-coupled receptor signaling pathway / G alpha (q) signalling events / Ras protein signal transduction / Extra-nuclear estrogen signaling / cell population proliferation / G protein-coupled receptor signaling pathway / lysosomal membrane / GTPase activity / synapse / protein-containing complex binding / signal transduction / extracellular exosome / membrane / plasma membrane / cytoplasm / cytosol Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
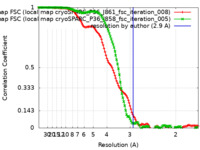
| Method | single particle reconstruction / cryo EM / Resolution: 2.9 Å | |||||||||
 Authors Authors | Barros-Alvarez X / Panova O / Skiniotis G | |||||||||
| Funding support | 1 items
| |||||||||
 Citation Citation |  Journal: Nature / Year: 2022 Journal: Nature / Year: 2022Title: The tethered peptide activation mechanism of adhesion GPCRs. Authors: Ximena Barros-Álvarez / Robert M Nwokonko / Alexander Vizurraga / Donna Matzov / Feng He / Makaía M Papasergi-Scott / Michael J Robertson / Ouliana Panova / Eliane Hadas Yardeni / Alpay B ...Authors: Ximena Barros-Álvarez / Robert M Nwokonko / Alexander Vizurraga / Donna Matzov / Feng He / Makaía M Papasergi-Scott / Michael J Robertson / Ouliana Panova / Eliane Hadas Yardeni / Alpay B Seven / Frank E Kwarcinski / Hongyu Su / Maria Claudia Peroto / Justin G Meyerowitz / Moran Shalev-Benami / Gregory G Tall / Georgios Skiniotis /   Abstract: Adhesion G-protein-coupled receptors (aGPCRs) are characterized by the presence of auto-proteolysing extracellular regions that are involved in cell-cell and cell-extracellular matrix interactions. ...Adhesion G-protein-coupled receptors (aGPCRs) are characterized by the presence of auto-proteolysing extracellular regions that are involved in cell-cell and cell-extracellular matrix interactions. Self cleavage within the aGPCR auto-proteolysis-inducing (GAIN) domain produces two protomers-N-terminal and C-terminal fragments-that remain non-covalently attached after receptors reach the cell surface. Upon dissociation of the N-terminal fragment, the C-terminus of the GAIN domain acts as a tethered agonist (TA) peptide to activate the seven-transmembrane domain with a mechanism that has been poorly understood. Here we provide cryo-electron microscopy snapshots of two distinct members of the aGPCR family, GPR56 (also known as ADGRG1) and latrophilin 3 (LPHN3 (also known as ADGRL3)). Low-resolution maps of the receptors in their N-terminal fragment-bound state indicate that the GAIN domain projects flexibly towards the extracellular space, keeping the encrypted TA peptide away from the seven-transmembrane domain. High-resolution structures of GPR56 and LPHN3 in their active, G-protein-coupled states, reveal that after dissociation of the extracellular region, the decrypted TA peptides engage the seven-transmembrane domain core with a notable conservation of interactions that also involve extracellular loop 2. TA binding stabilizes breaks in the middle of transmembrane helices 6 and 7 that facilitate aGPCR coupling and activation of heterotrimeric G proteins. Collectively, these results enable us to propose a general model for aGPCR activation. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_25076.map.gz emd_25076.map.gz | 1.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-25076-v30.xml emd-25076-v30.xml emd-25076.xml emd-25076.xml | 19.8 KB 19.8 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_25076_fsc.xml emd_25076_fsc.xml emd_25076_fsc_2.xml emd_25076_fsc_2.xml | 13.1 KB 13 KB | Display Display |  FSC data file FSC data file |
| Images |  emd_25076.png emd_25076.png | 48.4 KB | ||
| Filedesc metadata |  emd-25076.cif.gz emd-25076.cif.gz | 6.3 KB | ||
| Others |  emd_25076_additional_1.map.gz emd_25076_additional_1.map.gz emd_25076_additional_2.map.gz emd_25076_additional_2.map.gz | 844.3 KB 1.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-25076 http://ftp.pdbj.org/pub/emdb/structures/EMD-25076 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-25076 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-25076 | HTTPS FTP |
-Related structure data
| Related structure data |  7sf7MC  7sf8C C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_25076.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_25076.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Cryo-EM composite map for LPHN3 - miniG13 heterotrimer | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.8521 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: Local refinement of LPHN3 7TM domain
| File | emd_25076_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Local refinement of LPHN3 7TM domain | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Local refinement of miniG13 heterotrimer
| File | emd_25076_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Local refinement of miniG13 heterotrimer | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Latrophilin 3 (ADGRL3) 7TM domain bound to tethered agonist in co...
| Entire | Name: Latrophilin 3 (ADGRL3) 7TM domain bound to tethered agonist in complex with mini-G13 protein |
|---|---|
| Components |
|
-Supramolecule #1: Latrophilin 3 (ADGRL3) 7TM domain bound to tethered agonist in co...
| Supramolecule | Name: Latrophilin 3 (ADGRL3) 7TM domain bound to tethered agonist in complex with mini-G13 protein type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#4 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Isoform 1 of Adhesion G protein-coupled receptor L3
| Macromolecule | Name: Isoform 1 of Adhesion G protein-coupled receptor L3 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 34.313738 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: TNFAVLMAHV EVKHSDAVHD LLLDVITWVG ILLSLVCLLI CIFTFCFFRG LQSDRNTIHK NLCISLFVAE LLFLIGINRT DQPIACAVF AALLHFFFLA AFTWMFLEGV QLYIMLVEVF ESEHSRRKYF YLVGYGMPAL IVAVSAAVDY RSYGTDKVCW L RLDTYFIW ...String: TNFAVLMAHV EVKHSDAVHD LLLDVITWVG ILLSLVCLLI CIFTFCFFRG LQSDRNTIHK NLCISLFVAE LLFLIGINRT DQPIACAVF AALLHFFFLA AFTWMFLEGV QLYIMLVEVF ESEHSRRKYF YLVGYGMPAL IVAVSAAVDY RSYGTDKVCW L RLDTYFIW SFIGPATLII MLNVIFLGIA LYKMFHHTAI LKPESGCLDN IKSWVIGAIA LLCLLGLTWA FGLMYINEST VI MAYLFTI FNSLQGMFIF IFHCVLQKKV RKEYGKCLRT HCCSGKSTES SIGSGKTSGS LEVLFQ UniProtKB: Isoform 1 of Adhesion G protein-coupled receptor L3 |
-Macromolecule #2: G protein subunit 13 (Gi2-mini-G13 chimera)
| Macromolecule | Name: G protein subunit 13 (Gi2-mini-G13 chimera) / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 26.5744 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MGSTVSAEDK AAAERSKEID KCLSREKTYV KRLVKILLLG ADNSGKSTFL KQMRIIHGGS GGSGGTKGIH EYDFEIKNVP FKMVDVGGQ RSERKRWFEC FDSVTSILFL VDSSDFNRLT ESLNDFETIV NNRVFSNVSI ILFLNKTDLL EEKVQIVSIK D YFLEFEGD ...String: MGSTVSAEDK AAAERSKEID KCLSREKTYV KRLVKILLLG ADNSGKSTFL KQMRIIHGGS GGSGGTKGIH EYDFEIKNVP FKMVDVGGQ RSERKRWFEC FDSVTSILFL VDSSDFNRLT ESLNDFETIV NNRVFSNVSI ILFLNKTDLL EEKVQIVSIK D YFLEFEGD PHCLRDVQKF LVECFRNKRR DQQQKPLYHH FTTAINTENA RLIFRDVKDT ILHDNLKQLM LQ |
-Macromolecule #3: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 37.41693 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MSELDQLRQE AEQLKNQIRD ARKACADATL SQITNNIDPV GRIQMRTRRT LRGHLAKIYA MHWGTDSRLL VSASQDGKLI IWDSYTTNK VHAIPLRSSW VMTCAYAPSG NYVACGGLDN ICSIYNLKTR EGNVRVSREL AGHTGYLSCC RFLDDNQIVT S SGDTTCAL ...String: MSELDQLRQE AEQLKNQIRD ARKACADATL SQITNNIDPV GRIQMRTRRT LRGHLAKIYA MHWGTDSRLL VSASQDGKLI IWDSYTTNK VHAIPLRSSW VMTCAYAPSG NYVACGGLDN ICSIYNLKTR EGNVRVSREL AGHTGYLSCC RFLDDNQIVT S SGDTTCAL WDIETGQQTT TFTGHTGDVM SLSLAPDTRL FVSGACDASA KLWDVREGMC RQTFTGHESD INAICFFPNG NA FATGSDD ATCRLFDLRA DQELMTYSHD NIICGITSVS FSKSGRLLLA GYDDFNCNVW DALKADRAGV LAGHDNRVSC LGV TDDGMA VATGSWDSFL KIWN UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 |
-Macromolecule #4: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 7.861143 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MASNNTASIA QARKLVEQLK MEANIDRIKV SKAAADLMAY CEAHAKEDPL LTPVPASENP FREKKFFCAI L UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 |
-Macromolecule #5: water
| Macromolecule | Name: water / type: ligand / ID: 5 / Number of copies: 3 / Formula: HOH |
|---|---|
| Molecular weight | Theoretical: 18.015 Da |
| Chemical component information |  ChemComp-HOH: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 4.5 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Grid | Model: UltrAuFoil R1.2/1.3 |
| Vitrification | Cryogen name: ETHANE / Instrument: FEI VITROBOT MARK II |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 1.1 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 70.0 µm / Calibrated magnification: 57050 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller





















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)