[English] 日本語
 Yorodumi
Yorodumi- EMDB-25031: Synechocystis PCC 6803 Phycobilisome rod from OCP-PBS complex sample -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Synechocystis PCC 6803 Phycobilisome rod from OCP-PBS complex sample | |||||||||
 Map data Map data | B-factor sharpened map | |||||||||
 Sample Sample |
| |||||||||
| Function / homology |  Function and homology information Function and homology informationphycobilisome / plasma membrane-derived thylakoid membrane / photosynthesis Similarity search - Function | |||||||||
| Biological species |   | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.1 Å | |||||||||
 Authors Authors | Sauer PV / Sutter M / Dominguez-Martin MA / Kirst H / Kerfeld CA | |||||||||
| Funding support | European Union, 1 items
| |||||||||
 Citation Citation |  Journal: Nature / Year: 2022 Journal: Nature / Year: 2022Title: Structures of a phycobilisome in light-harvesting and photoprotected states. Authors: María Agustina Domínguez-Martín / Paul V Sauer / Henning Kirst / Markus Sutter / David Bína / Basil J Greber / Eva Nogales / Tomáš Polívka / Cheryl A Kerfeld /    Abstract: Phycobilisome (PBS) structures are elaborate antennae in cyanobacteria and red algae. These large protein complexes capture incident sunlight and transfer the energy through a network of embedded ...Phycobilisome (PBS) structures are elaborate antennae in cyanobacteria and red algae. These large protein complexes capture incident sunlight and transfer the energy through a network of embedded pigment molecules called bilins to the photosynthetic reaction centres. However, light harvesting must also be balanced against the risks of photodamage. A known mode of photoprotection is mediated by orange carotenoid protein (OCP), which binds to PBS when light intensities are high to mediate photoprotective, non-photochemical quenching. Here we use cryogenic electron microscopy to solve four structures of the 6.2 MDa PBS, with and without OCP bound, from the model cyanobacterium Synechocystis sp. PCC 6803. The structures contain a previously undescribed linker protein that binds to the membrane-facing side of PBS. For the unquenched PBS, the structures also reveal three different conformational states of the antenna, two previously unknown. The conformational states result from positional switching of two of the rods and may constitute a new mode of regulation of light harvesting. Only one of the three PBS conformations can bind to OCP, which suggests that not every PBS is equally susceptible to non-photochemical quenching. In the OCP-PBS complex, quenching is achieved through the binding of four 34 kDa OCPs organized as two dimers. The complex reveals the structure of the active form of OCP, in which an approximately 60 Å displacement of its regulatory carboxy terminal domain occurs. Finally, by combining our structure with spectroscopic properties, we elucidate energy transfer pathways within PBS in both the quenched and light-harvesting states. Collectively, our results provide detailed insights into the biophysical underpinnings of the control of cyanobacterial light harvesting. The data also have implications for bioengineering PBS regulation in natural and artificial light-harvesting systems. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_25031.map.gz emd_25031.map.gz | 167.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-25031-v30.xml emd-25031-v30.xml emd-25031.xml emd-25031.xml | 27.2 KB 27.2 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_25031.png emd_25031.png | 55.1 KB | ||
| Masks |  emd_25031_msk_1.map emd_25031_msk_1.map | 178 MB |  Mask map Mask map | |
| Others |  emd_25031_additional_1.map.gz emd_25031_additional_1.map.gz emd_25031_additional_2.map.gz emd_25031_additional_2.map.gz emd_25031_half_map_1.map.gz emd_25031_half_map_1.map.gz emd_25031_half_map_2.map.gz emd_25031_half_map_2.map.gz | 88.7 MB 157.6 MB 164.6 MB 164.6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-25031 http://ftp.pdbj.org/pub/emdb/structures/EMD-25031 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-25031 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-25031 | HTTPS FTP |
-Validation report
| Summary document |  emd_25031_validation.pdf.gz emd_25031_validation.pdf.gz | 785.2 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_25031_full_validation.pdf.gz emd_25031_full_validation.pdf.gz | 784.7 KB | Display | |
| Data in XML |  emd_25031_validation.xml.gz emd_25031_validation.xml.gz | 15.2 KB | Display | |
| Data in CIF |  emd_25031_validation.cif.gz emd_25031_validation.cif.gz | 17.9 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-25031 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-25031 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-25031 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-25031 | HTTPS FTP |
-Related structure data
| Related structure data |  7scaMC  7sc7C  7sc8C  7sc9C  7scbC  7sccC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_25031.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_25031.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | B-factor sharpened map | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.05 Å | ||||||||||||||||||||||||||||||||||||
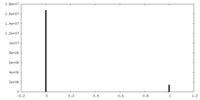
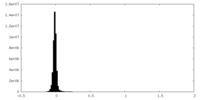
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_25031_msk_1.map emd_25031_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
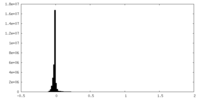
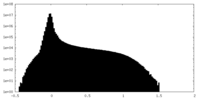
| Density Histograms |
-Additional map: 3D refinement
| File | emd_25031_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | 3D refinement | ||||||||||||
| Projections & Slices |
| ||||||||||||
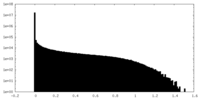
| Density Histograms |
-Additional map: DeepEMhancer sharpened map
| File | emd_25031_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | DeepEMhancer sharpened map | ||||||||||||
| Projections & Slices |
| ||||||||||||
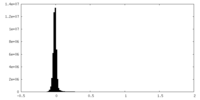
| Density Histograms |
-Half map: #2
| File | emd_25031_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_25031_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Complex of phycobilisome from Synechocystis PCC 6803 with OCP
| Entire | Name: Complex of phycobilisome from Synechocystis PCC 6803 with OCP |
|---|---|
| Components |
|
-Supramolecule #1: Complex of phycobilisome from Synechocystis PCC 6803 with OCP
| Supramolecule | Name: Complex of phycobilisome from Synechocystis PCC 6803 with OCP type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#6 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: C-phycocyanin alpha subunit
| Macromolecule | Name: C-phycocyanin alpha subunit / type: protein_or_peptide / ID: 1 / Number of copies: 18 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Strain: PCC 6803 / Kazusa |
| Molecular weight | Theoretical: 17.602529 KDa |
| Sequence | String: MKTPLTEAVS TADSQGRFLS STELQIAFGR LRQANAGLQA AKALTDNAQS LVNGAAQAVY NKFPYTTQTQ GNNFAADQRG KDKCARDIG YYLRIVTYCL VAGGTGPLDE YLIAGIDEIN RTFDLSPSWY VEALKYIKAN HGLSGDARDE ANSYLDYAIN A LS |
-Macromolecule #2: C-phycocyanin beta subunit
| Macromolecule | Name: C-phycocyanin beta subunit / type: protein_or_peptide / ID: 2 / Number of copies: 18 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Strain: PCC 6803 / Kazusa |
| Molecular weight | Theoretical: 18.142426 KDa |
| Sequence | String: MFDVFTRVVS QADARGEYLS GSQLDALSAT VAEGNKRIDS VNRITGNASA IVSNAARALF AEQPQLIQPG GNAYTSRRMA ACLRDMEII LRYVTYATFT GDASVLEDRC LNGLRETYVA LGVPGASVAA GVQKMKEAAL DIVNDPNGIT RGDCSAIVAE I AGYFDRAA AAVA |
-Macromolecule #3: Phycobilisome rod-core linker polypeptide CpcG
| Macromolecule | Name: Phycobilisome rod-core linker polypeptide CpcG / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Strain: PCC 6803 / Kazusa |
| Molecular weight | Theoretical: 28.938758 KDa |
| Sequence | String: MALPLLNYAP KSQNVRVEGY EIGSEEKPVV FTTENILSSS DMDNLIEAAY RQIFFHAFKW DREKVLESQL RNGQITVRDF VRGLLLSNT FRNSFYEKNS NYRFVEHCVQ KILGRDVYSE REKIAWSIVV ATKGYQGLID DLLNSDEYLN NFGYDTVPYQ R RRNLPGRE ...String: MALPLLNYAP KSQNVRVEGY EIGSEEKPVV FTTENILSSS DMDNLIEAAY RQIFFHAFKW DREKVLESQL RNGQITVRDF VRGLLLSNT FRNSFYEKNS NYRFVEHCVQ KILGRDVYSE REKIAWSIVV ATKGYQGLID DLLNSDEYLN NFGYDTVPYQ R RRNLPGRE AGELPFNIKS PRYDAYHRRQ LGFPQIVWQN EVRRFIPQEK KLTAGNPMNF LGMARSINPA ANTIPKVSAQ NI NIEASVP RR |
-Macromolecule #4: Phycobilisome 32.1 kDa linker polypeptide, phycocyanin-associated...
| Macromolecule | Name: Phycobilisome 32.1 kDa linker polypeptide, phycocyanin-associated, rod 1 type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Strain: PCC 6803 / Kazusa |
| Molecular weight | Theoretical: 32.558607 KDa |
| Sequence | String: MAITTAASRL GVAPYNESRP VELRPDFSLD DAKMVIRAVY RQVLGNDYIM DSERLKGAES LLTNGSISVR EFVRTVAKSE LYKKKFLYN NFQTRVIELN YKHLLGRAPF SEDEVIFHLD LYENQGFDAD IDSYIDSVEY QENFGENIVP YYRFNNQVGD R TVGFTRMF ...String: MAITTAASRL GVAPYNESRP VELRPDFSLD DAKMVIRAVY RQVLGNDYIM DSERLKGAES LLTNGSISVR EFVRTVAKSE LYKKKFLYN NFQTRVIELN YKHLLGRAPF SEDEVIFHLD LYENQGFDAD IDSYIDSVEY QENFGENIVP YYRFNNQVGD R TVGFTRMF RLYRGYANSD RSQLERSSSR LATELGQNTV SAIVGPSGSN AGWAYRPSRA GNTPAKALGG TVPFGQASKL FR VEITAIS APGYPKVRRS NKAVIVPFEQ LNQTLQQINR LGGKVASITP ASLS |
-Macromolecule #5: Phycobilisome 32.1 kDa linker polypeptide, phycocyanin-associated...
| Macromolecule | Name: Phycobilisome 32.1 kDa linker polypeptide, phycocyanin-associated, rod 2 type: protein_or_peptide / ID: 5 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Strain: PCC 6803 / Kazusa |
| Molecular weight | Theoretical: 30.836346 KDa |
| Sequence | String: MTSLVSAQRL GIVAVDEAIP LELRSRSTEE EVDAVILAVY RQVLGNDHLM SQERLTSAES LLRGREISVR DFVRAVALSE VYRQKFFHS NPQNRFIELN YKHLLGRAPY DQSEIAFHTD LYHQGGYEAE INSYIDSVEY TENFGDWVVP YFRGFATQRN Q KTVGFSRS ...String: MTSLVSAQRL GIVAVDEAIP LELRSRSTEE EVDAVILAVY RQVLGNDHLM SQERLTSAES LLRGREISVR DFVRAVALSE VYRQKFFHS NPQNRFIELN YKHLLGRAPY DQSEIAFHTD LYHQGGYEAE INSYIDSVEY TENFGDWVVP YFRGFATQRN Q KTVGFSRS FQVYRGYATS DRSQGNGSRS RLTRELARNT ASPVYAGSTA ESLRGTSAGS RNQMYRLQVI QGAAPGRGTR VR RGKAEYL VSYDNLSAKL QQINRQGDTV TMISLA |
-Macromolecule #6: Phycobilisome 8.9 kDa linker polypeptide, phycocyanin-associated, rod
| Macromolecule | Name: Phycobilisome 8.9 kDa linker polypeptide, phycocyanin-associated, rod type: protein_or_peptide / ID: 6 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Strain: PCC 6803 / Kazusa |
| Molecular weight | Theoretical: 9.333342 KDa |
| Sequence | String: MLGQSSLVGY SNTQAANRVF VYEVSGLRQT DANENSAHDI RRSGSVFIKV PYARMNDEMR RISRLGGTIV NIRPYQADSN EQN |
-Macromolecule #7: PHYCOCYANOBILIN
| Macromolecule | Name: PHYCOCYANOBILIN / type: ligand / ID: 7 / Number of copies: 54 / Formula: CYC |
|---|---|
| Molecular weight | Theoretical: 588.694 Da |
| Chemical component information | 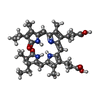 ChemComp-CYC: |
-Macromolecule #8: water
| Macromolecule | Name: water / type: ligand / ID: 8 / Number of copies: 1325 / Formula: HOH |
|---|---|
| Molecular weight | Theoretical: 18.015 Da |
| Chemical component information |  ChemComp-HOH: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 3.8 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Grid | Model: Quantifoil R2/1 / Material: COPPER / Mesh: 300 / Support film - Material: GOLD / Support film - topology: HOLEY |
| Vitrification | Cryogen name: ETHANE-PROPANE / Instrument: FEI VITROBOT MARK IV / Details: Manual blotting. |
- Electron microscopy
Electron microscopy
| Microscope | TFS KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: OTHER / Imaging mode: BRIGHT FIELD / Nominal defocus max: 1.6 µm / Nominal defocus min: 0.5 µm |
| Sample stage | Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: BACKBONE TRACE |
|---|---|
| Output model |  PDB-7sca: |
 Movie
Movie Controller
Controller












 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)