+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-24187 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of Mechanosensitive Ion Channel Flycatcher1 in GDN | |||||||||||||||
 Map data Map data | Structure of Mechanosensitive Ion Channel Flycatcher1 in GDN | |||||||||||||||
 Sample Sample |
| |||||||||||||||
 Keywords Keywords | mechanically activated ion channel / MEMBRANE PROTEIN | |||||||||||||||
| Biological species |  Dionaea muscipula (Venus flytrap) Dionaea muscipula (Venus flytrap) | |||||||||||||||
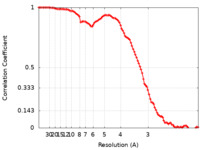
| Method | single particle reconstruction / cryo EM / Resolution: 2.8 Å | |||||||||||||||
 Authors Authors | Jojoa-Cruz S / Saotome K / Lee WH / Patapoutian A / Ward AB | |||||||||||||||
| Funding support |  United States, United States,  United Kingdom, 4 items United Kingdom, 4 items
| |||||||||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2022 Journal: Nat Commun / Year: 2022Title: Structural insights into the Venus flytrap mechanosensitive ion channel Flycatcher1. Authors: Sebastian Jojoa-Cruz / Kei Saotome / Che Chun Alex Tsui / Wen-Hsin Lee / Mark S P Sansom / Swetha E Murthy / Ardem Patapoutian / Andrew B Ward /   Abstract: Flycatcher1 (FLYC1), a MscS homolog, has recently been identified as a candidate mechanosensitive (MS) ion channel involved in Venus flytrap prey recognition. FLYC1 is a larger protein and its ...Flycatcher1 (FLYC1), a MscS homolog, has recently been identified as a candidate mechanosensitive (MS) ion channel involved in Venus flytrap prey recognition. FLYC1 is a larger protein and its sequence diverges from previously studied MscS homologs, suggesting it has unique structural features that contribute to its function. Here, we characterize FLYC1 by cryo-electron microscopy, molecular dynamics simulations, and electrophysiology. Akin to bacterial MscS and plant MSL1 channels, we find that FLYC1 central core includes side portals in the cytoplasmic cage that regulate ion preference and conduction, by identifying critical residues that modulate channel conductance. Topologically unique cytoplasmic flanking regions can adopt 'up' or 'down' conformations, making the channel asymmetric. Disruption of an up conformation-specific interaction severely delays channel deactivation by 40-fold likely due to stabilization of the channel open state. Our results illustrate novel structural features and likely conformational transitions that regulate mechano-gating of FLYC1. | |||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_24187.map.gz emd_24187.map.gz | 74.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-24187-v30.xml emd-24187-v30.xml emd-24187.xml emd-24187.xml | 20.4 KB 20.4 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_24187_fsc.xml emd_24187_fsc.xml | 9.9 KB | Display |  FSC data file FSC data file |
| Images |  emd_24187.png emd_24187.png | 94.5 KB | ||
| Filedesc metadata |  emd-24187.cif.gz emd-24187.cif.gz | 6.3 KB | ||
| Others |  emd_24187_half_map_1.map.gz emd_24187_half_map_1.map.gz emd_24187_half_map_2.map.gz emd_24187_half_map_2.map.gz | 65.4 MB 65.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-24187 http://ftp.pdbj.org/pub/emdb/structures/EMD-24187 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-24187 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-24187 | HTTPS FTP |
-Related structure data
| Related structure data |  7n5eMC  7n5dC  7n5fC  7n5gC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | |
| EM raw data |  EMPIAR-10740 (Title: Cryo-EM of Mechanosensitive Ion Channel Flycatcher1 in GDN EMPIAR-10740 (Title: Cryo-EM of Mechanosensitive Ion Channel Flycatcher1 in GDNData size: 5.7 TB Data #1: Unaligned multi-frame micrographs of FLYC1 in GDN, dataset 1 [micrographs - multiframe] Data #2: Unaligned multi-frame micrographs of FLYC1 in GDN, dataset 2 [micrographs - multiframe] Data #3: Gain references for dataset 1 and dataset 2 [micrographs - single frame]) |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_24187.map.gz / Format: CCP4 / Size: 83.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_24187.map.gz / Format: CCP4 / Size: 83.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Structure of Mechanosensitive Ion Channel Flycatcher1 in GDN | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.03 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Half map: Half map 1
| File | emd_24187_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map 1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map 2
| File | emd_24187_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map 2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : FLYC1
| Entire | Name: FLYC1 |
|---|---|
| Components |
|
-Supramolecule #1: FLYC1
| Supramolecule | Name: FLYC1 / type: organelle_or_cellular_component / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Dionaea muscipula (Venus flytrap) / Organ: Trap / Tissue: Trigger hair Dionaea muscipula (Venus flytrap) / Organ: Trap / Tissue: Trigger hair |
| Molecular weight | Theoretical: 607 KDa |
-Macromolecule #1: Mechanosensitive ion channel Flycatcher1
| Macromolecule | Name: Mechanosensitive ion channel Flycatcher1 / type: protein_or_peptide / ID: 1 / Number of copies: 7 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Dionaea muscipula (Venus flytrap) / Organ: Trap / Tissue: Trigger hair Dionaea muscipula (Venus flytrap) / Organ: Trap / Tissue: Trigger hair |
| Molecular weight | Theoretical: 86.814523 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MGSYLHEPPG DEPSMRIEQP KTADRAPEQV AIHICEPSKV VTESFPFSET AEPEAKSKNC PCPEIARIGP CPNKPPKIPI NRGLSRIST NKSRPKSRFG EPSWPVESSL DLTSQSPVSP YREEAFSVEN CGTAGSRRGS FARGTTSRAA SSSRKDETKE G PDEKEVYQ ...String: MGSYLHEPPG DEPSMRIEQP KTADRAPEQV AIHICEPSKV VTESFPFSET AEPEAKSKNC PCPEIARIGP CPNKPPKIPI NRGLSRIST NKSRPKSRFG EPSWPVESSL DLTSQSPVSP YREEAFSVEN CGTAGSRRGS FARGTTSRAA SSSRKDETKE G PDEKEVYQ RVTAQLSARN QKRMTVKLMI ELSVFLCLLG CLVCSLTVDG FKRYTVIGLD IWKWFLLLLV IFSGMLITHW IV HVAVFFV EWKFLMRKNV LYFTHGLKTS VEVFIWITVV LATWVMLIKP DVNQPHQTRK ILEFVTWTIV TVLIGAFLWL VKT TLLKIL ASSFHLNRFF DRIQESVFHH SVLQTLAGRP VVELAQGISR TESQDGAGQV SFMEHTKTQN KKVVDVGKLH QMKQ EKVPA WTMQLLVDVV SNSGLSTMSG MLDEDMVEGG VELDDDEITN EEQAIATAVR IFDNIVQDKV DQSYIDRVDL HRFLI WEEV DHLFPLFEVN EKGQISLKAF AKWVVKVYND QAALKHALND NKTAVKQLNK LVTAILIVMM IVIWLIVTGI ATTKLI VLL SSQLVVAAFI FGNTCKTIFE AIIFVFVMHP FDVGDRCVID GNKMLVEEMN ILTTVFLKWD KEKVYYPNSI LCTKAIG NF FRSPDQGDVL EFSVDFTTPV LKIGDLKDRI KMYLEQNLNF WHPQHNMVVK EIENVNKIKM ALFVNHTINF QDFAEKNR R RSELVLELKK IFEELDIKYN LLPQEISIRN MGSGSLEVLF Q |
-Macromolecule #2: PALMITIC ACID
| Macromolecule | Name: PALMITIC ACID / type: ligand / ID: 2 / Number of copies: 7 / Formula: PLM |
|---|---|
| Molecular weight | Theoretical: 256.424 Da |
| Chemical component information |  ChemComp-PLM: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 7 mg/mL | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 8 Component:
| ||||||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 283 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Digitization - Dimensions - Width: 3710 pixel / Digitization - Dimensions - Height: 3838 pixel / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 70.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.2 µm / Nominal defocus min: 0.7000000000000001 µm / Nominal magnification: 29000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: AB INITIO MODEL |
|---|---|
| Output model |  PDB-7n5e: |
 Movie
Movie Controller
Controller














 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)