[English] 日本語
 Yorodumi
Yorodumi- EMDB-15720: cryo-EM structure of carboxysome mini-shell: icosahedral structur... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
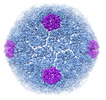
| Title | cryo-EM structure of carboxysome mini-shell: icosahedral structure from C1 construct (T=7) | |||||||||
 Map data Map data | main map | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Carboxysome / mini-shell / icosahedral / virus-like particle / STRUCTURAL PROTEIN | |||||||||
| Biological species |  Halothiobacillus neapolitanus (bacteria) Halothiobacillus neapolitanus (bacteria) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.29 Å | |||||||||
 Authors Authors | Ni T / Ng PC / Liu L / Zhang P | |||||||||
| Funding support |  United Kingdom, 2 items United Kingdom, 2 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2023 Journal: Nat Commun / Year: 2023Title: Intrinsically disordered CsoS2 acts as a general molecular thread for α-carboxysome shell assembly. Authors: Tao Ni / Qiuyao Jiang / Pei Cing Ng / Juan Shen / Hao Dou / Yanan Zhu / Julika Radecke / Gregory F Dykes / Fang Huang / Lu-Ning Liu / Peijun Zhang /   Abstract: Carboxysomes are a paradigm of self-assembling proteinaceous organelles found in nature, offering compartmentalisation of enzymes and pathways to enhance carbon fixation. In α-carboxysomes, the ...Carboxysomes are a paradigm of self-assembling proteinaceous organelles found in nature, offering compartmentalisation of enzymes and pathways to enhance carbon fixation. In α-carboxysomes, the disordered linker protein CsoS2 plays an essential role in carboxysome assembly and Rubisco encapsulation. Its mechanism of action, however, is not fully understood. Here we synthetically engineer α-carboxysome shells using minimal shell components and determine cryoEM structures of these to decipher the principle of shell assembly and encapsulation. The structures reveal that the intrinsically disordered CsoS2 C-terminus is well-structured and acts as a universal "molecular thread" stitching through multiple shell protein interfaces. We further uncover in CsoS2 a highly conserved repetitive key interaction motif, [IV]TG, which is critical to the shell assembly and architecture. Our study provides a general mechanism for the CsoS2-governed carboxysome shell assembly and cargo encapsulation and further advances synthetic engineering of carboxysomes for diverse biotechnological applications. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_15720.map.gz emd_15720.map.gz | 409.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-15720-v30.xml emd-15720-v30.xml emd-15720.xml emd-15720.xml | 13 KB 13 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_15720_fsc.xml emd_15720_fsc.xml | 19.9 KB | Display |  FSC data file FSC data file |
| Images |  emd_15720.png emd_15720.png | 253.6 KB | ||
| Others |  emd_15720_half_map_1.map.gz emd_15720_half_map_1.map.gz emd_15720_half_map_2.map.gz emd_15720_half_map_2.map.gz | 763.4 MB 763.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-15720 http://ftp.pdbj.org/pub/emdb/structures/EMD-15720 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-15720 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-15720 | HTTPS FTP |
-Validation report
| Summary document |  emd_15720_validation.pdf.gz emd_15720_validation.pdf.gz | 1.3 MB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_15720_full_validation.pdf.gz emd_15720_full_validation.pdf.gz | 1.3 MB | Display | |
| Data in XML |  emd_15720_validation.xml.gz emd_15720_validation.xml.gz | 28.2 KB | Display | |
| Data in CIF |  emd_15720_validation.cif.gz emd_15720_validation.cif.gz | 36.4 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-15720 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-15720 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-15720 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-15720 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_15720.map.gz / Format: CCP4 / Size: 824 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_15720.map.gz / Format: CCP4 / Size: 824 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | main map | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.831 Å | ||||||||||||||||||||||||||||||||||||
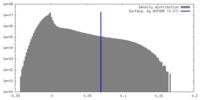
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: half1 map
| File | emd_15720_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half1 map | ||||||||||||
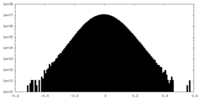
| Projections & Slices |
| ||||||||||||
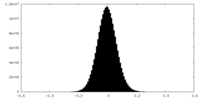
| Density Histograms |
-Half map: half2 map
| File | emd_15720_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half2 map | ||||||||||||
| Projections & Slices |
| ||||||||||||
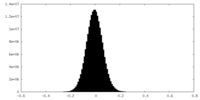
| Density Histograms |
- Sample components
Sample components
-Entire : mini-shell assembly from Halothiobacillus neapolitanus in icosahe...
| Entire | Name: mini-shell assembly from Halothiobacillus neapolitanus in icosahedral form |
|---|---|
| Components |
|
-Supramolecule #1: mini-shell assembly from Halothiobacillus neapolitanus in icosahe...
| Supramolecule | Name: mini-shell assembly from Halothiobacillus neapolitanus in icosahedral form type: complex / ID: 1 / Parent: 0 |
|---|---|
| Source (natural) | Organism:  Halothiobacillus neapolitanus (bacteria) Halothiobacillus neapolitanus (bacteria) |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8.1 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | TFS KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 40.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 3.0 µm / Nominal defocus min: 0.9 µm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller





















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)