+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 8pqf | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | c-KIT kinase domain in complex with avapritinib derivative 12 | |||||||||||||||
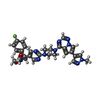
 Components Components | Mast/stem cell growth factor receptor Kit | |||||||||||||||
 Keywords Keywords | TRANSFERASE / c-KIT protein kinase / avapritinib / inhibitor / tyrosine kinase / transmembrane receptor / stem cell factor / SCF / stem cell factor receptor / GIST / gastrointestinal stromal tumors / P10721 | |||||||||||||||
| Function / homology |  Function and homology information Function and homology informationDasatinib-resistant KIT mutants / Imatinib-resistant KIT mutants / KIT mutants bind TKIs / Masitinib-resistant KIT mutants / Nilotinib-resistant KIT mutants / Regorafenib-resistant KIT mutants / Signaling by kinase domain mutants of KIT / Sunitinib-resistant KIT mutants / Signaling by juxtamembrane domain KIT mutants / Sorafenib-resistant KIT mutants ...Dasatinib-resistant KIT mutants / Imatinib-resistant KIT mutants / KIT mutants bind TKIs / Masitinib-resistant KIT mutants / Nilotinib-resistant KIT mutants / Regorafenib-resistant KIT mutants / Signaling by kinase domain mutants of KIT / Sunitinib-resistant KIT mutants / Signaling by juxtamembrane domain KIT mutants / Sorafenib-resistant KIT mutants / Signaling by extracellular domain mutants of KIT / hematopoietic stem cell migration / melanocyte adhesion / positive regulation of pyloric antrum smooth muscle contraction / positive regulation of colon smooth muscle contraction / stem cell factor receptor activity / positive regulation of vascular associated smooth muscle cell differentiation / melanocyte migration / Kit signaling pathway / tongue development / : / positive regulation of dendritic cell cytokine production / positive regulation of small intestine smooth muscle contraction / mast cell chemotaxis / positive regulation of mast cell proliferation / mast cell differentiation / Fc receptor signaling pathway / glycosphingolipid metabolic process / mast cell proliferation / positive regulation of long-term neuronal synaptic plasticity / positive regulation of pseudopodium assembly / positive regulation of mast cell cytokine production / detection of mechanical stimulus involved in sensory perception of sound / lymphoid progenitor cell differentiation / melanocyte differentiation / immature B cell differentiation / germ cell migration / erythropoietin-mediated signaling pathway / myeloid progenitor cell differentiation / digestive tract development / negative regulation of programmed cell death / embryonic hemopoiesis / lamellipodium assembly / positive regulation of tyrosine phosphorylation of STAT protein / Regulation of KIT signaling / megakaryocyte development / growth factor binding / stem cell population maintenance / cytokine binding / mast cell degranulation / positive regulation of Notch signaling pathway / pigmentation / negative regulation of reproductive process / negative regulation of developmental process / hemopoiesis / T cell differentiation / somatic stem cell population maintenance / spermatid development / response to cadmium ion / ectopic germ cell programmed cell death / hematopoietic progenitor cell differentiation / : / ovarian follicle development / TFAP2 (AP-2) family regulates transcription of growth factors and their receptors / transmembrane receptor protein tyrosine kinase activity / Transcriptional and post-translational regulation of MITF-M expression and activity / acrosomal vesicle / SH2 domain binding / B cell differentiation / Signaling by phosphorylated juxtamembrane, extracellular and kinase domain KIT mutants / epithelial cell proliferation / stem cell differentiation / erythrocyte differentiation / cell chemotaxis / positive regulation of receptor signaling pathway via JAK-STAT / Signaling by SCF-KIT / receptor protein-tyrosine kinase / visual learning / male gonad development / cytoplasmic side of plasma membrane / fibrillar center / cytokine-mediated signaling pathway / Constitutive Signaling by Aberrant PI3K in Cancer / cell-cell junction / cell migration / PIP3 activates AKT signaling / regulation of cell shape / regulation of cell population proliferation / protein autophosphorylation / PI5P, PP2A and IER3 Regulate PI3K/AKT Signaling / RAF/MAP kinase cascade / actin cytoskeleton organization / protease binding / protein tyrosine kinase activity / spermatogenesis / receptor complex / positive regulation of phosphatidylinositol 3-kinase/protein kinase B signal transduction / positive regulation of MAPK cascade / intracellular signal transduction / positive regulation of cell migration Similarity search - Function | |||||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.9 Å MOLECULAR REPLACEMENT / Resolution: 1.9 Å | |||||||||||||||
 Authors Authors | Teuber, A. / Mueller, M.P. / Rauh, D. | |||||||||||||||
| Funding support | European Union,  Germany, 4items Germany, 4items
| |||||||||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2024 Journal: Nat Commun / Year: 2024Title: Avapritinib-based SAR studies unveil a binding pocket in KIT and PDGFRA. Authors: Teuber, A. / Schulz, T. / Fletcher, B.S. / Gontla, R. / Muhlenberg, T. / Zischinsky, M.L. / Niggenaber, J. / Weisner, J. / Kleinbolting, S.B. / Lategahn, J. / Sievers, S. / Muller, M.P. / Bauer, S. / Rauh, D. | |||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  8pqf.cif.gz 8pqf.cif.gz | 303.8 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb8pqf.ent.gz pdb8pqf.ent.gz | 203.4 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  8pqf.json.gz 8pqf.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/pq/8pqf https://data.pdbj.org/pub/pdb/validation_reports/pq/8pqf ftp://data.pdbj.org/pub/pdb/validation_reports/pq/8pqf ftp://data.pdbj.org/pub/pdb/validation_reports/pq/8pqf | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  8pq9C  8pqaC  8pqbC  8pqcC  8pqdC  8pqeC  8pqgC  8pqhC  8pqiC  8pqjC  8pqkC C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
| Experimental dataset #1 | Data reference:  10.18430/m38pqf / Data set type: diffraction image data / Details: https://www.proteindiffraction.org/project/8PQF/ 10.18430/m38pqf / Data set type: diffraction image data / Details: https://www.proteindiffraction.org/project/8PQF/ |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||||||
| 2 | 
| ||||||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 37194.430 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: KIT, SCFR / Production host: Homo sapiens (human) / Gene: KIT, SCFR / Production host:  References: UniProt: P10721, receptor protein-tyrosine kinase #2: Chemical | #3: Water | ChemComp-HOH / | Has ligand of interest | Y | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 1.9 Å3/Da / Density % sol: 35.35 % |
|---|---|
| Crystal grow | Temperature: 285.15 K / Method: vapor diffusion, hanging drop / Details: 3 mg/mL, 21% PEG3350, 125 mM Li3-citrate |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  SLS SLS  / Beamline: X10SA / Wavelength: 1 Å / Beamline: X10SA / Wavelength: 1 Å |
| Detector | Type: DECTRIS EIGER2 X 16M / Detector: PIXEL / Date: Feb 10, 2023 |
| Radiation | Monochromator: Si(111) / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1 Å / Relative weight: 1 |
| Reflection | Resolution: 1.9→47.96 Å / Num. obs: 53195 / % possible obs: 100 % / Redundancy: 13.36 % / Biso Wilson estimate: 40.35 Å2 / CC1/2: 0.999 / Rrim(I) all: 0.094 / Net I/σ(I): 14.58 |
| Reflection shell | Resolution: 1.9→2 Å / Redundancy: 12.86 % / Mean I/σ(I) obs: 1.53 / Num. unique obs: 7488 / CC1/2: 0.738 / Rrim(I) all: 1.597 / % possible all: 100 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT / Resolution: 1.9→43 Å / SU ML: 0.2274 / Cross valid method: FREE R-VALUE / σ(F): 1.35 / Phase error: 25.2202 MOLECULAR REPLACEMENT / Resolution: 1.9→43 Å / SU ML: 0.2274 / Cross valid method: FREE R-VALUE / σ(F): 1.35 / Phase error: 25.2202 Stereochemistry target values: GeoStd + Monomer Library + CDL v1.2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.1 Å / Solvent model: FLAT BULK SOLVENT MODEL | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 47.66 Å2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.9→43 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group | Refine-ID: X-RAY DIFFRACTION
|
 Movie
Movie Controller
Controller



 PDBj
PDBj