+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7ydq | |||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of PfNT1(Y190A)-GFP in complex with GSK4 | |||||||||||||||||||||||||||||||||||||||
 Components Components | Nucleoside transporter 1,Green fluorescent protein | |||||||||||||||||||||||||||||||||||||||
 Keywords Keywords | MEMBRANE PROTEIN / malaria / nucleoside transporter / GSK4 / TRANSPORT PROTEIN | |||||||||||||||||||||||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationadenosine transport / nucleoside transmembrane transporter activity / purine nucleobase transport / bioluminescence / generation of precursor metabolites and energy / plasma membrane Similarity search - Function | |||||||||||||||||||||||||||||||||||||||
| Biological species |   | |||||||||||||||||||||||||||||||||||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 4.04 Å | |||||||||||||||||||||||||||||||||||||||
 Authors Authors | Wang, C. / Yu, L.Y. / Li, J.L. / Ren, R.B. / Deng, D. | |||||||||||||||||||||||||||||||||||||||
| Funding support |  China, 2items China, 2items
| |||||||||||||||||||||||||||||||||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2023 Journal: Nat Commun / Year: 2023Title: Structural basis of the substrate recognition and inhibition mechanism of Plasmodium falciparum nucleoside transporter PfENT1. Authors: Chen Wang / Leiye Yu / Jiying Zhang / Yanxia Zhou / Bo Sun / Qingjie Xiao / Minhua Zhang / Huayi Liu / Jinhong Li / Jialu Li / Yunzi Luo / Jie Xu / Zhong Lian / Jingwen Lin / Xiang Wang / ...Authors: Chen Wang / Leiye Yu / Jiying Zhang / Yanxia Zhou / Bo Sun / Qingjie Xiao / Minhua Zhang / Huayi Liu / Jinhong Li / Jialu Li / Yunzi Luo / Jie Xu / Zhong Lian / Jingwen Lin / Xiang Wang / Peng Zhang / Li Guo / Ruobing Ren / Dong Deng /  Abstract: By lacking de novo purine biosynthesis enzymes, Plasmodium falciparum requires purine nucleoside uptake from host cells. The indispensable nucleoside transporter ENT1 of P. falciparum facilitates ...By lacking de novo purine biosynthesis enzymes, Plasmodium falciparum requires purine nucleoside uptake from host cells. The indispensable nucleoside transporter ENT1 of P. falciparum facilitates nucleoside uptake in the asexual blood stage. Specific inhibitors of PfENT1 prevent the proliferation of P. falciparum at submicromolar concentrations. However, the substrate recognition and inhibitory mechanism of PfENT1 are still elusive. Here, we report cryo-EM structures of PfENT1 in apo, inosine-bound, and inhibitor-bound states. Together with in vitro binding and uptake assays, we identify that inosine is the primary substrate of PfENT1 and that the inosine-binding site is located in the central cavity of PfENT1. The endofacial inhibitor GSK4 occupies the orthosteric site of PfENT1 and explores the allosteric site to block the conformational change of PfENT1. Furthermore, we propose a general "rocker switch" alternating access cycle for ENT transporters. Understanding the substrate recognition and inhibitory mechanisms of PfENT1 will greatly facilitate future efforts in the rational design of antimalarial drugs. | |||||||||||||||||||||||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7ydq.cif.gz 7ydq.cif.gz | 142 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7ydq.ent.gz pdb7ydq.ent.gz | 89.6 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  7ydq.json.gz 7ydq.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/yd/7ydq https://data.pdbj.org/pub/pdb/validation_reports/yd/7ydq ftp://data.pdbj.org/pub/pdb/validation_reports/yd/7ydq ftp://data.pdbj.org/pub/pdb/validation_reports/yd/7ydq | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  33756MC  7wn0C  7wn1C M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
| #1: Protein | Mass: 77035.766 Da / Num. of mol.: 1 / Mutation: Y190A Source method: isolated from a genetically manipulated source Details: PfNT1(Y190A) fused with GFP the GFP was inserted into PfNT1 between K370 and K371 Source: (gene. exp.)   Gene: PF3D7_1347200, GFP / Cell line (production host): IPLB-Sf-21-AE(Sf9) / Production host:  |
|---|---|
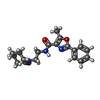
| #2: Chemical | ChemComp-IRX / |
| Has ligand of interest | Y |
| Has protein modification | N |
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: nucleoside/nucleobase transporter fusion with GFP / Type: COMPLEX Details: the cDNA of the GFP was cloned into PfNT1 between K370 and K371 Entity ID: #1 / Source: RECOMBINANT | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular weight | Value: 47.5 kDa/nm / Experimental value: NO | ||||||||||||
| Source (natural) |
| ||||||||||||
| Source (recombinant) | Organism:  | ||||||||||||
| Buffer solution | pH: 6 | ||||||||||||
| Specimen | Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES | ||||||||||||
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD / Nominal defocus max: 1800 nm / Nominal defocus min: 1100 nm |
| Image recording | Electron dose: 52.452 e/Å2 / Film or detector model: GATAN K2 SUMMIT (4k x 4k) |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EM software | Name: PHENIX / Category: model refinement | ||||||||||||||||||||||||
| CTF correction | Type: NONE | ||||||||||||||||||||||||
| 3D reconstruction | Resolution: 4.04 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 329768 / Symmetry type: POINT | ||||||||||||||||||||||||
| Refinement | Cross valid method: NONE Stereochemistry target values: GeoStd + Monomer Library + CDL v1.2 | ||||||||||||||||||||||||
| Displacement parameters | Biso mean: 171.65 Å2 | ||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller





 PDBj
PDBj