+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-7544 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Human CLC-1 chloride ion channel, transmembrane domain | |||||||||
 Map data Map data | Summed map, filtered and sharpened | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | chloride / channel / CLC / TRANSPORT PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationvoltage-gated chloride channel activity / neuronal action potential propagation / chloride transport / chloride channel complex / T-tubule / muscle contraction / chloride transmembrane transport / Stimuli-sensing channels / protein homodimerization activity / plasma membrane Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.36 Å | |||||||||
 Authors Authors | Park E / MacKinnon R | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Elife / Year: 2018 Journal: Elife / Year: 2018Title: Structure of the CLC-1 chloride channel from . Authors: Eunyong Park / Roderick MacKinnon /  Abstract: CLC channels mediate passive Cl conduction, while CLC transporters mediate active Cl transport coupled to H transport in the opposite direction. The distinction between CLC-0/1/2 channels and CLC ...CLC channels mediate passive Cl conduction, while CLC transporters mediate active Cl transport coupled to H transport in the opposite direction. The distinction between CLC-0/1/2 channels and CLC transporters seems undetectable by amino acid sequence. To understand why they are different functionally we determined the structure of the human CLC-1 channel. Its 'glutamate gate' residue, known to mediate proton transfer in CLC transporters, adopts a location in the structure that appears to preclude it from its transport function. Furthermore, smaller side chains produce a wider pore near the intracellular surface, potentially reducing a kinetic barrier for Cl conduction. When the corresponding residues are mutated in a transporter, it is converted to a channel. Finally, Cl at key sites in the pore appear to interact with reduced affinity compared to transporters. Thus, subtle differences in glutamate gate conformation, internal pore diameter and Cl affinity distinguish CLC channels and transporters. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_7544.map.gz emd_7544.map.gz | 117.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-7544-v30.xml emd-7544-v30.xml emd-7544.xml emd-7544.xml | 15.2 KB 15.2 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_7544_fsc.xml emd_7544_fsc.xml | 11.4 KB | Display |  FSC data file FSC data file |
| Images |  emd_7544.png emd_7544.png | 131.6 KB | ||
| Filedesc metadata |  emd-7544.cif.gz emd-7544.cif.gz | 5.6 KB | ||
| Others |  emd_7544_half_map_1.map.gz emd_7544_half_map_1.map.gz emd_7544_half_map_2.map.gz emd_7544_half_map_2.map.gz | 96.9 MB 96.9 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-7544 http://ftp.pdbj.org/pub/emdb/structures/EMD-7544 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-7544 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-7544 | HTTPS FTP |
-Validation report
| Summary document |  emd_7544_validation.pdf.gz emd_7544_validation.pdf.gz | 895.8 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_7544_full_validation.pdf.gz emd_7544_full_validation.pdf.gz | 895.4 KB | Display | |
| Data in XML |  emd_7544_validation.xml.gz emd_7544_validation.xml.gz | 18.3 KB | Display | |
| Data in CIF |  emd_7544_validation.cif.gz emd_7544_validation.cif.gz | 23.9 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-7544 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-7544 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-7544 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-7544 | HTTPS FTP |
-Related structure data
| Related structure data |  6coyMC  7545C  6cozC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_7544.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_7544.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Summed map, filtered and sharpened | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.03 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
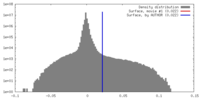
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Half map: Half map 1, unfiltered
| File | emd_7544_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map 1, unfiltered | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map 2, unfiltered
| File | emd_7544_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map 2, unfiltered | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Human CLC-1 chloride ion channel
| Entire | Name: Human CLC-1 chloride ion channel |
|---|---|
| Components |
|
-Supramolecule #1: Human CLC-1 chloride ion channel
| Supramolecule | Name: Human CLC-1 chloride ion channel / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Chloride channel protein 1
| Macromolecule | Name: Chloride channel protein 1 / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 108.733172 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MEQSRSQQRG GEQSWWGSDP QYQYMPFEHC TSYGLPSENG GLQHRLRKDA GPRHNVHPTQ IYGHHKEQFS DREQDIGMPK KTGSSSTVD SKDEDHYSKC QDCIHRLGQV VRRKLGEDGI FLVLLGLLMA LVSWSMDYVS AKSLQAYKWS YAQMQPSLPL Q FLVWVTFP ...String: MEQSRSQQRG GEQSWWGSDP QYQYMPFEHC TSYGLPSENG GLQHRLRKDA GPRHNVHPTQ IYGHHKEQFS DREQDIGMPK KTGSSSTVD SKDEDHYSKC QDCIHRLGQV VRRKLGEDGI FLVLLGLLMA LVSWSMDYVS AKSLQAYKWS YAQMQPSLPL Q FLVWVTFP LVLILFSALF CHLISPQAVG SGIPEMKTIL RGVVLKEYLT MKAFVAKVVA LTAGLGSGIP VGKEGPFVHI AS ICAAVLS KFMSVFCGVY EQPYYYSDIL TVGCAVGVGC CFGTPLGGVL FSIEVTSTYF AVRNYWRGFF AATFSAFVFR VLA VWNKDA VTITALFRTN FRMDFPFDLK ELPAFAAIGI CCGLLGAVFV YLHRQVMLGV RKHKALSQFL AKHRLLYPGI VTFV IASFT FPPGMGQFMA GELMPREAIS TLFDNNTWVK HAGDPESLGQ SAVWIHPRVN VVIIIFLFFV MKFWMSIVAT TMPIP CGGF MPVFVLGAAF GRLVGEIMAM LFPDGILFDD IIYKILPGGY AVIGAAALTG AVSHTVSTAV ICFELTGQIA HILPMM VAV ILANMVAQSL QPSLYDSIIQ VKKLPYLPDL GWNQLSKYTI FVEDIMVRDV KFVSASYTYG ELRTLLQTTT VKTLPLV DS KDSMILLGSV ERSELQALLQ RHLCPERRLR AAQEMARKLS ELPYDGKARL AGEGLPGAPP GRPESFAFVD EDEDEDLS G KSELPPSLAL HPSTTAPLSP EEPNGPLPGH KQQPEAPEPA GQRPSIFQSL LHCLLGRARP TKKKTTQDST DLVDNMSPE EIEAWEQEQL SQPVCFDSCC IDQSPFQLVE QTTLHKTHTL FSLLGLHLAY VTSMGKLRGV LALEELQKAI EGHTKSGVQL RPPLASFRN TTSTRKSTGA PPSSAENWNL PEDRPGATGT GDVIAASPET PVPSPSPEPP LSLAPGKVEG ELEELELVES P GLEEELAD ILQGPSLRST DEEDEDELIL UniProtKB: Chloride channel protein 1 |
-Macromolecule #2: CHLORIDE ION
| Macromolecule | Name: CHLORIDE ION / type: ligand / ID: 2 / Number of copies: 4 / Formula: CL |
|---|---|
| Molecular weight | Theoretical: 35.453 Da |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 39.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: OTHER |
|---|---|
| Output model |  PDB-6coy: |
 Movie
Movie Controller
Controller















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)