+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4hm8 | ||||||
|---|---|---|---|---|---|---|---|
| Title | Naphthalene 1,2-Dioxygenase bound to thioanisole | ||||||
 Components Components | (Naphthalene 1,2-dioxygenase subunit ...) x 2 | ||||||
 Keywords Keywords | Oxidoreductase/Oxidoreductase inhibitor / Oxidoreductase-Oxidoreductase inhibitor complex | ||||||
| Function / homology |  Function and homology information Function and homology informationnaphthalene 1,2-dioxygenase / naphthalene 1,2-dioxygenase activity / 3-phenylpropionate catabolic process / catabolic process / dioxygenase activity / 2 iron, 2 sulfur cluster binding / iron ion binding Similarity search - Function | ||||||
| Biological species |  Pseudomonas sp. C18 (bacteria) Pseudomonas sp. C18 (bacteria) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.3 Å MOLECULAR REPLACEMENT / Resolution: 1.3 Å | ||||||
 Authors Authors | Ferraro, D.J. / Ramaswamy, S. | ||||||
 Citation Citation |  Journal: Iucrj / Year: 2017 Journal: Iucrj / Year: 2017Title: One enzyme, many reactions: structural basis for the various reactions catalyzed by naphthalene 1,2-dioxygenase. Authors: Ferraro, D.J. / Okerlund, A. / Brown, E. / Ramaswamy, S. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4hm8.cif.gz 4hm8.cif.gz | 288.7 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4hm8.ent.gz pdb4hm8.ent.gz | 229.4 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4hm8.json.gz 4hm8.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/hm/4hm8 https://data.pdbj.org/pub/pdb/validation_reports/hm/4hm8 ftp://data.pdbj.org/pub/pdb/validation_reports/hm/4hm8 ftp://data.pdbj.org/pub/pdb/validation_reports/hm/4hm8 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  4hjlC  4hkvC  4hm0C  4hm2C  4hm3C  4hm4C  4hm5C  4hm6C  4hm7C C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||||||
| Unit cell |
| ||||||||||||
| Components on special symmetry positions |
| ||||||||||||
| Details | THE BIOLOGICAL ASSEMBLY IS AND ALPHA3 BETA3 HEXAMER. ONE ALPHA-BETA DIMER IS IN THE ASSYMETRIC UNIT. THE OTHERS CAN BE GENERATED BY THE THREE-FOLD AXIS: Y, -X-Y, Z -X-Y, X, Z |
- Components
Components
-Naphthalene 1,2-dioxygenase subunit ... , 2 types, 2 molecules AB
| #1: Protein | Mass: 49664.355 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Pseudomonas sp. C18 (bacteria) / Strain: NCIB 9816-4 / Gene: doxB, NC_004999.1 / Plasmid: pDTG121 / Production host: Pseudomonas sp. C18 (bacteria) / Strain: NCIB 9816-4 / Gene: doxB, NC_004999.1 / Plasmid: pDTG121 / Production host:  |
|---|---|
| #2: Protein | Mass: 22969.088 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Pseudomonas sp. C18 (bacteria) / Strain: NCIB 9816-4 / Gene: doxD, NC_004999.1 / Plasmid: pDTG121 / Production host: Pseudomonas sp. C18 (bacteria) / Strain: NCIB 9816-4 / Gene: doxD, NC_004999.1 / Plasmid: pDTG121 / Production host:  |
-Non-polymers , 6 types, 986 molecules 



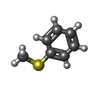






| #3: Chemical | ChemComp-EDO / #4: Chemical | ChemComp-SO4 / #5: Chemical | ChemComp-FES / | #6: Chemical | ChemComp-FE / | #7: Chemical | ChemComp-16R / ( | #8: Water | ChemComp-HOH / | |
|---|
-Details
| Has protein modification | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.71 Å3/Da / Density % sol: 54.67 % |
|---|---|
| Crystal grow | Temperature: 279.15 K / Method: vapor diffusion / pH: 5 Details: 1.9-2.2 M AMMONIUM SULFATE, 4-6%, DIOXANE, 0.1 M MES, pH 5.0, VAPOR DIFFUSION, temperature 279.15K |
-Data collection
| Diffraction |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ALS ALS  / Beamline: 4.2.2 / Wavelength: 1.04 Å / Beamline: 4.2.2 / Wavelength: 1.04 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Detector | Type: NOIR-1 / Detector: CCD / Date: Nov 1, 2004 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Radiation |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Radiation wavelength | Wavelength: 1.04 Å / Relative weight: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection | Resolution: 1.3→14.99 Å / Num. obs: 190595 / % possible obs: 99.4 % / Redundancy: 3.99 % / Rmerge(I) obs: 0.083 / Χ2: 1 / Net I/σ(I): 8.1 / Scaling rejects: 5754 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection shell | Diffraction-ID: 1,2
|
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT / Resolution: 1.3→14.988 Å / Occupancy max: 1 / Occupancy min: 0.32 / FOM work R set: 0.8602 / SU ML: 0.17 / σ(F): 0.63 / Phase error: 21.58 / Stereochemistry target values: ML MOLECULAR REPLACEMENT / Resolution: 1.3→14.988 Å / Occupancy max: 1 / Occupancy min: 0.32 / FOM work R set: 0.8602 / SU ML: 0.17 / σ(F): 0.63 / Phase error: 21.58 / Stereochemistry target values: ML
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 74.55 Å2 / Biso mean: 16.8508 Å2 / Biso min: 6.65 Å2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.3→14.988 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Refine-ID: X-RAY DIFFRACTION / Total num. of bins used: 30
|
 Movie
Movie Controller
Controller














 PDBj
PDBj













