[English] 日本語
 Yorodumi
Yorodumi- EMDB-4114: Israeli acute paralysis virus heated to 63 degree - full particle -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-4114 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Israeli acute paralysis virus heated to 63 degree - full particle | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | IAPV / Dicistroviridae / full particle / Virus | |||||||||
| Function / homology |  Function and homology information Function and homology information | |||||||||
| Biological species |  Israeli acute paralysis virus Israeli acute paralysis virus | |||||||||
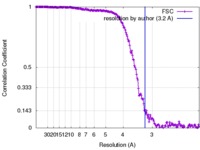
| Method | single particle reconstruction / cryo EM / Resolution: 3.2 Å | |||||||||
 Authors Authors | Mullapudi E / Fuzik T | |||||||||
| Funding support |  Czech Republic, Czech Republic,  Germany, 2 items Germany, 2 items
| |||||||||
 Citation Citation |  Journal: J Virol / Year: 2017 Journal: J Virol / Year: 2017Title: Cryo-electron Microscopy Study of the Genome Release of the Dicistrovirus Israeli Acute Bee Paralysis Virus. Authors: Edukondalu Mullapudi / Tibor Füzik / Antonín Přidal / Pavel Plevka /  Abstract: Viruses of the family Dicistroviridae can cause substantial economic damage by infecting agriculturally important insects. Israeli acute bee paralysis virus (IAPV) causes honeybee colony collapse ...Viruses of the family Dicistroviridae can cause substantial economic damage by infecting agriculturally important insects. Israeli acute bee paralysis virus (IAPV) causes honeybee colony collapse disorder in the United States. High-resolution molecular details of the genome delivery mechanism of dicistroviruses are unknown. Here we present a cryo-electron microscopy analysis of IAPV virions induced to release their genomes in vitro We determined structures of full IAPV virions primed to release their genomes to a resolution of 3.3 Å and of empty capsids to a resolution of 3.9 Å. We show that IAPV does not form expanded A particles before genome release as in the case of related enteroviruses of the family Picornaviridae The structural changes observed in the empty IAPV particles include detachment of the VP4 minor capsid proteins from the inner face of the capsid and partial loss of the structure of the N-terminal arms of the VP2 capsid proteins. Unlike the case for many picornaviruses, the empty particles of IAPV are not expanded relative to the native virions and do not contain pores in their capsids that might serve as channels for genome release. Therefore, rearrangement of a unique region of the capsid is probably required for IAPV genome release. IMPORTANCE: Honeybee populations in Europe and North America are declining due to pressure from pathogens, including viruses. Israeli acute bee paralysis virus (IAPV), a member of the family ...IMPORTANCE: Honeybee populations in Europe and North America are declining due to pressure from pathogens, including viruses. Israeli acute bee paralysis virus (IAPV), a member of the family Dicistroviridae, causes honeybee colony collapse disorder in the United States. The delivery of virus genomes into host cells is necessary for the initiation of infection. Here we present a structural cryo-electron microscopy analysis of IAPV particles induced to release their genomes. We show that genome release is not preceded by an expansion of IAPV virions as in the case of related picornaviruses that infect vertebrates. Furthermore, minor capsid proteins detach from the capsid upon genome release. The genome leaves behind empty particles that have compact protein shells. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_4114.map.gz emd_4114.map.gz | 39.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-4114-v30.xml emd-4114-v30.xml emd-4114.xml emd-4114.xml | 16.7 KB 16.7 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_4114_fsc.xml emd_4114_fsc.xml | 20.1 KB | Display |  FSC data file FSC data file |
| Images |  emd_4114.png emd_4114.png | 309.7 KB | ||
| Filedesc metadata |  emd-4114.cif.gz emd-4114.cif.gz | 6 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-4114 http://ftp.pdbj.org/pub/emdb/structures/EMD-4114 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-4114 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-4114 | HTTPS FTP |
-Related structure data
| Related structure data |  5lwgMC  4115C  5lwiC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_4114.map.gz / Format: CCP4 / Size: 137.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_4114.map.gz / Format: CCP4 / Size: 137.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.07 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Israeli acute paralysis virus
| Entire | Name:  Israeli acute paralysis virus Israeli acute paralysis virus |
|---|---|
| Components |
|
-Supramolecule #1: Israeli acute paralysis virus
| Supramolecule | Name: Israeli acute paralysis virus / type: virus / ID: 1 / Parent: 0 / Macromolecule list: all / NCBI-ID: 294365 / Sci species name: Israeli acute paralysis virus / Virus type: VIRION / Virus isolate: OTHER / Virus enveloped: No / Virus empty: No |
|---|---|
| Host (natural) | Organism:  |
-Macromolecule #1: VP1
| Macromolecule | Name: VP1 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Israeli acute paralysis virus Israeli acute paralysis virus |
| Molecular weight | Theoretical: 23.776541 KDa |
| Sequence | String: INIGNKTNEN VISFFDSTDA ETQNHNALMK GCGEFIVNLR TLLRTFRTIT DNWILQANTK TPITDLTNTT DAQGRDYMSY LSYLYRFYR GGRRYKFFNT TPLKQSQTCY IRSFLIPRNY SADEINVDGP SHITYPVINP VHEVEVPFYS QYRKIPIAST S DKGYDSSL ...String: INIGNKTNEN VISFFDSTDA ETQNHNALMK GCGEFIVNLR TLLRTFRTIT DNWILQANTK TPITDLTNTT DAQGRDYMSY LSYLYRFYR GGRRYKFFNT TPLKQSQTCY IRSFLIPRNY SADEINVDGP SHITYPVINP VHEVEVPFYS QYRKIPIAST S DKGYDSSL MYFSNTATTQ IVARAGNDDF TFGWMIGPPQ LQGESRSVAP UniProtKB: Structural polyprotein |
-Macromolecule #2: VP3
| Macromolecule | Name: VP3 / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Israeli acute paralysis virus Israeli acute paralysis virus |
| Molecular weight | Theoretical: 33.260754 KDa |
| Sequence | String: SKPRNQQQVC PLQNVPAWGY SLYKGIDMSV PLAYDPNNEL GDLKDVFPSA VDEMAIGYVC GNPAVKHVLT WKTTDAIQKP IANGDDWGG VIPVGMPCYS KSIRTTRISA TENRETEVMD AAPCEYVANM FSYWRATMCY RITVVKTAFH TGRLEIFFEP G VIPVKPTV ...String: SKPRNQQQVC PLQNVPAWGY SLYKGIDMSV PLAYDPNNEL GDLKDVFPSA VDEMAIGYVC GNPAVKHVLT WKTTDAIQKP IANGDDWGG VIPVGMPCYS KSIRTTRISA TENRETEVMD AAPCEYVANM FSYWRATMCY RITVVKTAFH TGRLEIFFEP G VIPVKPTV NNIGPDQDRL TGAVAPSDNN YKYILDLTND TEVTIRVPFV SNKMFLKTAG IYGANSENNW NFHESFSGFL CI RPVTKLM APDTVSDNVS IVVWKWAEDV VVVEPKPLTS GPTQVYRPPP TASTAVEVLN VEL UniProtKB: Structural polyprotein |
-Macromolecule #3: VP2
| Macromolecule | Name: VP2 / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Israeli acute paralysis virus Israeli acute paralysis virus |
| Molecular weight | Theoretical: 27.637932 KDa |
| Sequence | String: NVHNTELASS TSENSVETQE ITTFHDVETP NRIDTPMAQD TSSARNMDDT HSIIQFLQRP VLIDNIEIIA GTTADANKPL SRYVLDQQN SQKYVRSWTL PSTVLRAGGK AQKLANFKYL RCDVQVKLVL NANPFVAGRM YLAYSPYDDK VDTARSVLQT S RAGVTGYP ...String: NVHNTELASS TSENSVETQE ITTFHDVETP NRIDTPMAQD TSSARNMDDT HSIIQFLQRP VLIDNIEIIA GTTADANKPL SRYVLDQQN SQKYVRSWTL PSTVLRAGGK AQKLANFKYL RCDVQVKLVL NANPFVAGRM YLAYSPYDDK VDTARSVLQT S RAGVTGYP GVELDFQLDN SVEMTIPYAS FQEAYDLVTG TEDFVQLYLF PITPVLGPKS ESESSKVDIS VYMWLSNISL VI PTYRINP UniProtKB: Structural polyprotein |
-Macromolecule #4: VP4
| Macromolecule | Name: VP4 / type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Israeli acute paralysis virus Israeli acute paralysis virus |
| Molecular weight | Theoretical: 5.899765 KDa |
| Sequence | String: TSENPKIGPI SEVASGVKTT ANGIERIPVI GEIAKPVTTA VKWFADVVGT VAAIFGW UniProtKB: Structural polyprotein |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Grid | Model: Quantifoil R2/1 / Material: COPPER / Mesh: 200 / Support film - Material: CARBON / Support film - topology: HOLEY |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 298 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON II (4k x 4k) / Detector mode: INTEGRATING / Digitization - Frames/image: 1-7 / Number grids imaged: 1 / Number real images: 1500 / Average exposure time: 1.0 sec. / Average electron dose: 21.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 100.0 µm / Calibrated defocus max: 3.0 µm / Calibrated defocus min: 1.0 µm / Calibrated magnification: 75000 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 3.0 µm / Nominal defocus min: 1.0 µm / Nominal magnification: 75000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Details | initial rigid body fit was done by Chimera, then the model was refined using realspace refinement in Phenix and finaly reciprocal refined in REFMAC. |
|---|---|
| Refinement | Space: RECIPROCAL / Protocol: RIGID BODY FIT |
| Output model |  PDB-5lwg: |
 Movie
Movie Controller
Controller













 Y (Sec.)
Y (Sec.) X (Row.)
X (Row.) Z (Col.)
Z (Col.)