[English] 日本語
 Yorodumi
Yorodumi- EMDB-40807: BG505 Boost2 SOSIP.664 in complex with NHP polyclonal antibody Base1 -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | BG505 Boost2 SOSIP.664 in complex with NHP polyclonal antibody Base1 | ||||||||||||
 Map data Map data | main map | ||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | HIV-1 / Env / NHP / Antibody / Polyclonal / VIRAL PROTEIN | ||||||||||||
| Biological species |   Human immunodeficiency virus 1 Human immunodeficiency virus 1 | ||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 4.2 Å | ||||||||||||
 Authors Authors | Pratap PP / Antansijevic A / Ward AB | ||||||||||||
| Funding support |  United States, 3 items United States, 3 items
| ||||||||||||
 Citation Citation |  Journal: bioRxiv / Year: 2023 Journal: bioRxiv / Year: 2023Title: Focusing antibody responses to the fusion peptide in rhesus macaques. Authors: Christopher A Cottrell / Payal P Pratap / Kimberly M Cirelli / Diane G Carnathan / Chiamaka A Enemuo / Aleksandar Antanasijevic / Gabriel Ozorowski / Leigh M Sewall / Hongmei Gao / Kelli M ...Authors: Christopher A Cottrell / Payal P Pratap / Kimberly M Cirelli / Diane G Carnathan / Chiamaka A Enemuo / Aleksandar Antanasijevic / Gabriel Ozorowski / Leigh M Sewall / Hongmei Gao / Kelli M Greene / Joel D Allen / Julia T Ngo / Yury Choe / Bartek Nogal / Murillo Silva / Jinal Bhiman / Matthias Pauthner / Darrell J Irvine / David Montefiori / Max Crispin / Dennis R Burton / Guido Silvestri / Shane Crotty / Andrew B Ward /    Abstract: Immunodominance of antibodies targeting non-neutralizing epitopes and the high level of somatic hypermutation within germinal centers (GCs) required for most HIV broadly neutralizing antibodies ...Immunodominance of antibodies targeting non-neutralizing epitopes and the high level of somatic hypermutation within germinal centers (GCs) required for most HIV broadly neutralizing antibodies (bnAbs) are major impediments to the development of an effective HIV vaccine. Rational protein vaccine design and non-conventional immunization strategies are potential avenues to overcome these hurdles. Here, we report using implantable osmotic pumps to continuously deliver a series of epitope-targeted immunogens to rhesus macaques over the course of six months to elicit immune responses against the conserved fusion peptide. Antibody specificities and GC responses were tracked longitudinally using electron microscopy polyclonal epitope mapping (EMPEM) and lymph node fine-needle aspirates, respectively. Application of cryoEMPEM delineated key residues for on-target and off-target responses that can drive the next round of structure-based vaccine design. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_40807.map.gz emd_40807.map.gz | 166.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-40807-v30.xml emd-40807-v30.xml emd-40807.xml emd-40807.xml | 17.5 KB 17.5 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_40807_fsc.xml emd_40807_fsc.xml | 12.8 KB | Display |  FSC data file FSC data file |
| Images |  emd_40807.png emd_40807.png | 121.7 KB | ||
| Masks |  emd_40807_msk_1.map emd_40807_msk_1.map | 178 MB |  Mask map Mask map | |
| Others |  emd_40807_half_map_1.map.gz emd_40807_half_map_1.map.gz emd_40807_half_map_2.map.gz emd_40807_half_map_2.map.gz | 141 MB 140.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-40807 http://ftp.pdbj.org/pub/emdb/structures/EMD-40807 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-40807 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-40807 | HTTPS FTP |
-Validation report
| Summary document |  emd_40807_validation.pdf.gz emd_40807_validation.pdf.gz | 1 MB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_40807_full_validation.pdf.gz emd_40807_full_validation.pdf.gz | 1 MB | Display | |
| Data in XML |  emd_40807_validation.xml.gz emd_40807_validation.xml.gz | 20 KB | Display | |
| Data in CIF |  emd_40807_validation.cif.gz emd_40807_validation.cif.gz | 26.3 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-40807 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-40807 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-40807 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-40807 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_40807.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_40807.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | main map | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.031 Å | ||||||||||||||||||||||||||||||||||||
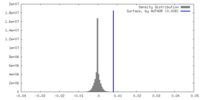
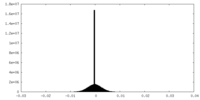
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_40807_msk_1.map emd_40807_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
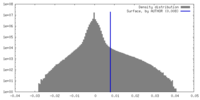
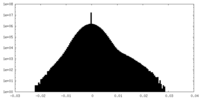
| Density Histograms |
-Half map: half map 2
| File | emd_40807_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map 2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: half map1
| File | emd_40807_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : BG505 Boost 2 in complex with NHP Polyclonal Antibody Base1
| Entire | Name: BG505 Boost 2 in complex with NHP Polyclonal Antibody Base1 |
|---|---|
| Components |
|
-Supramolecule #1: BG505 Boost 2 in complex with NHP Polyclonal Antibody Base1
| Supramolecule | Name: BG505 Boost 2 in complex with NHP Polyclonal Antibody Base1 type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:   Human immunodeficiency virus 1 Human immunodeficiency virus 1 |
| Molecular weight | Theoretical: 470 KDa |
-Macromolecule #1: BG505 Boost 2 SOSIP gp120
| Macromolecule | Name: BG505 Boost 2 SOSIP gp120 / type: protein_or_peptide / ID: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Human immunodeficiency virus 1 Human immunodeficiency virus 1 |
| Sequence | String: MDAMKRGLCC VLLLCGAVFV SPSQEIHARF RRGARAENLW VTVYYGVPVW KDAETTLFCA SDAKAYETKK HNVWATHCCV PTDPNPQEIH LENVTEEFNM WKNNMVEQMH TDIISLWDQS LKPCVKLTPL CVTLQCTNVT NNITDDMRGE LKNCSFNMTT ELRDKKQKVY ...String: MDAMKRGLCC VLLLCGAVFV SPSQEIHARF RRGARAENLW VTVYYGVPVW KDAETTLFCA SDAKAYETKK HNVWATHCCV PTDPNPQEIH LENVTEEFNM WKNNMVEQMH TDIISLWDQS LKPCVKLTPL CVTLQCTNVT NNITDDMRGE LKNCSFNMTT ELRDKKQKVY SLFYRLDVVQ INENQGNRSN NSNKEYRLIN CNTSAITQAC PKVSFEPIPI HYCAPAGFAI LKCKDKKFNG TGPCTNVSTV QCTHGIKPVV STQLLLNGSL AEEEVIIRSE NITNNAKNIL VQLNESVQIN CTRPNNNTRK SIRIGPGQWF YATGDIIGDI RQAHCNVSKA TWNETLGKVV KQLRKHFGNN TIIRFANSSG GDLEVTTHSF NCGGEFFYCN TSGLFNSTWI SNTSVQGSNS TGSNDSITLP CRIKQIINMW QRIGQAMYAP PIQGVIRCVS NITGLILTRD GGSTNSTTET FRPGGGDMRD NWRSELYKYK VVKIEPLGVA PTRCKRRVVG RRRRRR |
-Macromolecule #2: BG505 Boost 2 SOSIP gp41
| Macromolecule | Name: BG505 Boost 2 SOSIP gp41 / type: protein_or_peptide / ID: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Human immunodeficiency virus 1 Human immunodeficiency virus 1 |
| Sequence | String: AVGIGAVFLG FLGAAGSTMG AASMTLTVQA RNLLSGIVQQ QSNLLRAPEC QQHLLKLTVW GIKQLQARVL AVERYLRDQQ LLGIWGCSGK LICCTNVPWN STWSNRNLSE IWDNMTWLQW DKEISNYTQI IYGLLEESQN QQEKNEQDLL ALD |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 Component:
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Grid | Model: UltrAuFoil R1.2/1.3 / Material: GOLD / Mesh: 300 / Pretreatment - Type: PLASMA CLEANING | ||||||||
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Number real images: 7339 / Average electron dose: 41.3591843915 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.0 µm / Nominal defocus min: 0.5 µm / Nominal magnification: 22500 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller











 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)