+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | cryo-EM structure of hSlo1 in plasma membrane vesicles | |||||||||
 Map data Map data | cryo-EM map of hSlo1 in plasma membrane vesicles, sharpened with a B-factor of 59.1 | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Slo1 / BK channel / Ca2+- and voltage-activated K+ channel / ion channel / plasma membrane vesicles / TRANSPORT PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationAcetylcholine inhibits contraction of outer hair cells / micturition / large conductance calcium-activated potassium channel activity / Ca2+ activated K+ channels / calcium-activated potassium channel activity / negative regulation of cell volume / smooth muscle contraction involved in micturition / response to carbon monoxide / response to osmotic stress / Sensory processing of sound by inner hair cells of the cochlea ...Acetylcholine inhibits contraction of outer hair cells / micturition / large conductance calcium-activated potassium channel activity / Ca2+ activated K+ channels / calcium-activated potassium channel activity / negative regulation of cell volume / smooth muscle contraction involved in micturition / response to carbon monoxide / response to osmotic stress / Sensory processing of sound by inner hair cells of the cochlea / cGMP effects / intracellular potassium ion homeostasis / voltage-gated potassium channel activity / voltage-gated potassium channel complex / potassium ion transmembrane transport / regulation of membrane potential / response to calcium ion / caveola / potassium ion transport / vasodilation / actin binding / postsynaptic membrane / response to hypoxia / apical plasma membrane / positive regulation of apoptotic process / metal ion binding / identical protein binding / membrane / plasma membrane Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.7 Å | |||||||||
 Authors Authors | Tao X / Zhao C / MacKinnon R | |||||||||
| Funding support |  United States, 2 items United States, 2 items
| |||||||||
 Citation Citation |  Journal: Proc Natl Acad Sci U S A / Year: 2023 Journal: Proc Natl Acad Sci U S A / Year: 2023Title: Membrane protein isolation and structure determination in cell-derived membrane vesicles. Authors: Xiao Tao / Chen Zhao / Roderick MacKinnon /  Abstract: Integral membrane protein structure determination traditionally requires extraction from cell membranes using detergents or polymers. Here, we describe the isolation and structure determination of ...Integral membrane protein structure determination traditionally requires extraction from cell membranes using detergents or polymers. Here, we describe the isolation and structure determination of proteins in membrane vesicles derived directly from cells. Structures of the ion channel Slo1 from total cell membranes and from cell plasma membranes were determined at 3.8 Å and 2.7 Å resolution, respectively. The plasma membrane environment stabilizes Slo1, revealing an alteration of global helical packing, polar lipid, and cholesterol interactions that stabilize previously unresolved regions of the channel and an additional ion binding site in the Ca regulatory domain. The two methods presented enable structural analysis of both internal and plasma membrane proteins without disrupting weakly interacting proteins, lipids, and cofactors that are essential to biological function. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_40044.map.gz emd_40044.map.gz | 258.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-40044-v30.xml emd-40044-v30.xml emd-40044.xml emd-40044.xml | 23.6 KB 23.6 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_40044_fsc.xml emd_40044_fsc.xml | 13.7 KB | Display |  FSC data file FSC data file |
| Images |  emd_40044.png emd_40044.png | 219.9 KB | ||
| Filedesc metadata |  emd-40044.cif.gz emd-40044.cif.gz | 7.3 KB | ||
| Others |  emd_40044_additional_1.map.gz emd_40044_additional_1.map.gz emd_40044_half_map_1.map.gz emd_40044_half_map_1.map.gz emd_40044_half_map_2.map.gz emd_40044_half_map_2.map.gz | 133.7 MB 254.5 MB 254.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-40044 http://ftp.pdbj.org/pub/emdb/structures/EMD-40044 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-40044 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-40044 | HTTPS FTP |
-Validation report
| Summary document |  emd_40044_validation.pdf.gz emd_40044_validation.pdf.gz | 1.2 MB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_40044_full_validation.pdf.gz emd_40044_full_validation.pdf.gz | 1.2 MB | Display | |
| Data in XML |  emd_40044_validation.xml.gz emd_40044_validation.xml.gz | 23 KB | Display | |
| Data in CIF |  emd_40044_validation.cif.gz emd_40044_validation.cif.gz | 29.7 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-40044 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-40044 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-40044 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-40044 | HTTPS FTP |
-Related structure data
| Related structure data |  8ghfMC  8gh9C  8ghgC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_40044.map.gz / Format: CCP4 / Size: 274.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_40044.map.gz / Format: CCP4 / Size: 274.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | cryo-EM map of hSlo1 in plasma membrane vesicles, sharpened with a B-factor of 59.1 | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.743 Å | ||||||||||||||||||||||||||||||||||||
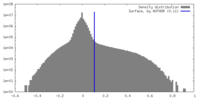
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: unsharpened cryo-EM map of hSlo1 in plasma membrane vesicles
| File | emd_40044_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | unsharpened cryo-EM map of hSlo1 in plasma membrane vesicles | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: half map 1 of hSlo1 in plasma membrane vesicles
| File | emd_40044_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map 1 of hSlo1 in plasma membrane vesicles | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: half map 2 of hSlo1 in plasma membrane vesicles
| File | emd_40044_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map 2 of hSlo1 in plasma membrane vesicles | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : ALFA-hSlo1-eGFP ion channel
| Entire | Name: ALFA-hSlo1-eGFP ion channel |
|---|---|
| Components |
|
-Supramolecule #1: ALFA-hSlo1-eGFP ion channel
| Supramolecule | Name: ALFA-hSlo1-eGFP ion channel / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Calcium-activated potassium channel subunit alpha-1
| Macromolecule | Name: Calcium-activated potassium channel subunit alpha-1 / type: protein_or_peptide / ID: 1 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 122.440102 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MAPSRLEEEL RRRLTEPDAL IIPVTMEVPC DSRGQRMWWA FLASSMVTFF GGLFIILLWR TLKYLWTVCC HCGGKTKEAQ KINNGSSQA DGTLKPVDEK EEAVAAEVGW MTSVKDWAGV MISAQTLTGR VLVVLVFALS IGALVIYFID SSNPIESCQN F YKDFTLQI ...String: MAPSRLEEEL RRRLTEPDAL IIPVTMEVPC DSRGQRMWWA FLASSMVTFF GGLFIILLWR TLKYLWTVCC HCGGKTKEAQ KINNGSSQA DGTLKPVDEK EEAVAAEVGW MTSVKDWAGV MISAQTLTGR VLVVLVFALS IGALVIYFID SSNPIESCQN F YKDFTLQI DMAFNVFFLL YFGLRFIAAN DKLWFWLEVN SVVDFFTVPP VFVSVYLNRS WLGLRFLRAL RLIQFSEILQ FL NILKTSN SIKLVNLLSI FISTWLTAAG FIHLVENSGD PWENFQNNQA LTYWECVYLL MVTMSTVGYG DVYAKTTLGR LFM VFFILG GLAMFASYVP EIIELIGNRK KYGGSYSAVS GRKHIVVCGH ITLESVSNFL KDFLHKDRDD VNVEIVFLHN ISPN LELEA LFKRHFTQVE FYQGSVLNPH DLARVKIESA DACLILANKY CADPDAEDAS NIMRVISIKN YHPKIRIITQ MLQYH NKAH LLNIPSWNWK EGDDAICLAE LKLGFIAQSC LAQGLSTMLA NLFSMRSFIK IEEDTWQKYY LEGVSNEMYT EYLSSA FVG LSFPTVCELC FVKLKLLMIA IEYKSANRES RILINPGNHL KIQEGTLGFF IASDAKEVKR AFFYCKACHD DITDPKR IK KCGCKRLEDE QPSTLSPKKK QRNGGMRNSP NTSPKLMRHD PLLIPGNDQI DNMDSNVKKY DSTGMFHWCA PKEIEKVI L TRSEAAMTVL SGHVVVCIFG DVSSALIGLR NLVMPLRASN FHYHELKHIV FVGSIEYLKR EWETLHNFPK VSILPGTPL SRADLRAVNI NLCDMCVILS ANQNNIDDTS LQDKECILAS LNIKSMQFDD SIGVLQANSQ GFTPPGMDRS SPDNSPVHGM LRQPSITTG VNIPIITELV NDTNVQFLDQ DDDDDPDTEL YLTQPFACGT AFAVSVLDSL MSATYFNDNI LTLIRTLVTG G ATPELEAL IAEENALRGG YSTPQTLANR DRCRVAQLAL LDGPFADLGD GGCYGDLFCK ALKTYNMLCF GIYRLRDAHL ST PSQCTKR YVITNPPYEF ELVPTDLIFC LMQFD(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK) UniProtKB: Calcium-activated potassium channel subunit alpha-1 |
-Macromolecule #2: MAGNESIUM ION
| Macromolecule | Name: MAGNESIUM ION / type: ligand / ID: 2 / Number of copies: 4 / Formula: MG |
|---|---|
| Molecular weight | Theoretical: 24.305 Da |
-Macromolecule #3: CALCIUM ION
| Macromolecule | Name: CALCIUM ION / type: ligand / ID: 3 / Number of copies: 8 / Formula: CA |
|---|---|
| Molecular weight | Theoretical: 40.078 Da |
-Macromolecule #4: (2S)-3-(hexadecanoyloxy)-2-[(9Z)-octadec-9-enoyloxy]propyl 2-(tri...
| Macromolecule | Name: (2S)-3-(hexadecanoyloxy)-2-[(9Z)-octadec-9-enoyloxy]propyl 2-(trimethylammonio)ethyl phosphate type: ligand / ID: 4 / Number of copies: 88 / Formula: POV |
|---|---|
| Molecular weight | Theoretical: 760.076 Da |
| Chemical component information |  ChemComp-POV: |
-Macromolecule #5: CHOLESTEROL
| Macromolecule | Name: CHOLESTEROL / type: ligand / ID: 5 / Number of copies: 24 / Formula: CLR |
|---|---|
| Molecular weight | Theoretical: 386.654 Da |
| Chemical component information |  ChemComp-CLR: |
-Macromolecule #6: SODIUM ION
| Macromolecule | Name: SODIUM ION / type: ligand / ID: 6 / Number of copies: 4 |
|---|---|
| Molecular weight | Theoretical: 22.99 Da |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Grid | Model: Quantifoil R1.2/1.3 / Material: GOLD / Mesh: 400 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 22 sec. |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 295.15 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Energy filter - Slit width: 6 eV |
| Image recording | Film or detector model: FEI FALCON IV (4k x 4k) / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.0 µm / Nominal defocus min: 1.0 µm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller









 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)