[English] 日本語
 Yorodumi
Yorodumi- EMDB-3849: Single particle cryo-STEM of ferritin with Fe at low stoichiometry -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-3849 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Single particle cryo-STEM of ferritin with Fe at low stoichiometry | |||||||||
 Map data Map data | Ferritin with 100 Fe atoms from single particle cryo-STEM data | |||||||||
 Sample Sample |
| |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 21.0 Å | |||||||||
 Authors Authors | Elad N / Bellapadrona G / Houben L / Sagi I / Elbaum M | |||||||||
 Citation Citation |  Journal: Proc Natl Acad Sci U S A / Year: 2017 Journal: Proc Natl Acad Sci U S A / Year: 2017Title: Detection of isolated protein-bound metal ions by single-particle cryo-STEM. Authors: Nadav Elad / Giuliano Bellapadrona / Lothar Houben / Irit Sagi / Michael Elbaum /  Abstract: Metal ions play essential roles in many aspects of biological chemistry. Detecting their presence and location in proteins and cells is important for understanding biological function. Conventional ...Metal ions play essential roles in many aspects of biological chemistry. Detecting their presence and location in proteins and cells is important for understanding biological function. Conventional structural methods such as X-ray crystallography and cryo-transmission electron microscopy can identify metal atoms on protein only if the protein structure is solved to atomic resolution. We demonstrate here the detection of isolated atoms of Zn and Fe on ferritin, using cryogenic annular dark-field scanning transmission electron microscopy (cryo-STEM) coupled with single-particle 3D reconstructions. Zn atoms are found in a pattern that matches precisely their location at the ferroxidase sites determined earlier by X-ray crystallography. By contrast, the Fe distribution is smeared along an arc corresponding to the proposed path from the ferroxidase sites to the mineral nucleation sites along the twofold axes. In this case the single-particle reconstruction is interpreted as a probability distribution function based on the average of individual locations. These results establish conditions for detection of isolated metal atoms in the broader context of electron cryo-microscopy and tomography. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_3849.map.gz emd_3849.map.gz | 925 KB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-3849-v30.xml emd-3849-v30.xml emd-3849.xml emd-3849.xml | 12.2 KB 12.2 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_3849.png emd_3849.png | 76.8 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-3849 http://ftp.pdbj.org/pub/emdb/structures/EMD-3849 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-3849 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-3849 | HTTPS FTP |
-Validation report
| Summary document |  emd_3849_validation.pdf.gz emd_3849_validation.pdf.gz | 216.8 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_3849_full_validation.pdf.gz emd_3849_full_validation.pdf.gz | 215.9 KB | Display | |
| Data in XML |  emd_3849_validation.xml.gz emd_3849_validation.xml.gz | 4.3 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-3849 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-3849 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-3849 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-3849 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_3849.map.gz / Format: CCP4 / Size: 2 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_3849.map.gz / Format: CCP4 / Size: 2 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Ferritin with 100 Fe atoms from single particle cryo-STEM data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 3.3 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
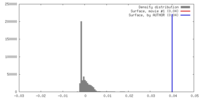
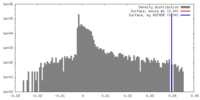
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Human heavy chain ferritin with Fe at low stoichiometry
| Entire | Name: Human heavy chain ferritin with Fe at low stoichiometry |
|---|---|
| Components |
|
-Supramolecule #1: Human heavy chain ferritin with Fe at low stoichiometry
| Supramolecule | Name: Human heavy chain ferritin with Fe at low stoichiometry type: complex / ID: 1 / Parent: 0 Details: Bacterially expressed and purified human heavy chain ferritin mixed with a 100 fold molar excess of Fe(II)SO4 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Recombinant expression | Organism:  |
| Molecular weight | Theoretical: 504 KDa |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.13 mg/mL | ||||||
|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.2 / Component:
| ||||||
| Grid | Model: Quantifoil R2/1 / Material: COPPER / Mesh: 200 / Support film - #0 - Film type ID: 1 / Support film - #0 - Material: CARBON / Support film - #0 - topology: HOLEY / Support film - #1 - Film type ID: 2 / Support film - #1 - Material: CARBON / Support film - #1 - topology: CONTINUOUS / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Atmosphere: AIR | ||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 95 % / Chamber temperature: 297 K / Instrument: LEICA EM GP | ||||||
| Details | For metal loading, stock protein (2.9 mg/ml) was diluted to 0.25 mg/ml and mixed with a 100 fold molar excess of Fe(II)SO4. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TECNAI F20 |
|---|---|
| Details | STEM mode imaging using Fischione model 3000 HAADF detector. Camera length: 520 mm. |
| Image recording | Film or detector model: OTHER / Digitization - Dimensions - Width: 2048 pixel / Digitization - Dimensions - Height: 2048 pixel / Number grids imaged: 1 / Number real images: 58 / Average electron dose: 127.0 e/Å2 Details: ADF images collected with a Fischione Model 3000 high-angle annular DF detector |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 70.0 µm / Illumination mode: OTHER / Imaging mode: OTHER / Cs: 2.0 mm / Nominal magnification: 450000 |
| Sample stage | Specimen holder model: GATAN 626 SINGLE TILT LIQUID NITROGEN CRYO TRANSFER HOLDER Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Tecnai F20 / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller




 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)