+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of BIRC6/HtrA2-S306A | |||||||||
 Map data Map data | main map | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Ubiquitin / E3 ligase / Apoptosis / Autophagy / IAP / LIGASE | |||||||||
| Function / homology |  Function and homology information Function and homology informationHtrA2 peptidase / pentacyclic triterpenoid metabolic process / : / spongiotrophoblast layer development / ceramide metabolic process / regulation of autophagy of mitochondrion / mitochondrial protein catabolic process / CD40 receptor complex / labyrinthine layer development / ALK mutants bind TKIs ...HtrA2 peptidase / pentacyclic triterpenoid metabolic process / : / spongiotrophoblast layer development / ceramide metabolic process / regulation of autophagy of mitochondrion / mitochondrial protein catabolic process / CD40 receptor complex / labyrinthine layer development / ALK mutants bind TKIs / programmed cell death / positive regulation of extrinsic apoptotic signaling pathway in absence of ligand / Flemming body / protein serine/threonine kinase inhibitor activity / serine-type endopeptidase complex / microtubule organizing center / adult walking behavior / positive regulation of protein targeting to mitochondrion / response to herbicide / cysteine-type endopeptidase inhibitor activity / execution phase of apoptosis / : / positive regulation of execution phase of apoptosis / ubiquitin conjugating enzyme activity / protein autoprocessing / ubiquitin ligase inhibitor activity / negative regulation of cell cycle / regulation of multicellular organism growth / neuron development / cellular response to interferon-beta / negative regulation of oxidative stress-induced intrinsic apoptotic signaling pathway / cellular response to retinoic acid / forebrain development / Mitochondrial protein degradation / serine-type peptidase activity / regulation of cytokinesis / mitochondrion organization / negative regulation of extrinsic apoptotic signaling pathway / mitochondrial membrane / trans-Golgi network / RING-type E3 ubiquitin transferase / protein catabolic process / cellular response to growth factor stimulus / mitochondrial intermembrane space / spindle pole / cytoplasmic side of plasma membrane / intrinsic apoptotic signaling pathway in response to DNA damage / ubiquitin-protein transferase activity / unfolded protein binding / Signaling by ALK fusions and activated point mutants / peptidase activity / regulation of cell population proliferation / cellular response to heat / cellular response to oxidative stress / midbody / neuron apoptotic process / negative regulation of neuron apoptotic process / cell population proliferation / cytoskeleton / intracellular signal transduction / endosome / protein ubiquitination / positive regulation of apoptotic process / protein phosphorylation / cell division / serine-type endopeptidase activity / centrosome / positive regulation of cell population proliferation / endoplasmic reticulum membrane / chromatin / negative regulation of apoptotic process / apoptotic process / endoplasmic reticulum / mitochondrion / proteolysis / identical protein binding / membrane / nucleus / cytosol Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.21 Å | |||||||||
 Authors Authors | Hunkeler M / Fischer ES | |||||||||
| Funding support |  United States, 2 items United States, 2 items
| |||||||||
 Citation Citation |  Journal: Science / Year: 2023 Journal: Science / Year: 2023Title: Structures of BIRC6-client complexes provide a mechanism of SMAC-mediated release of caspases. Authors: Moritz Hunkeler / Cyrus Y Jin / Eric S Fischer /  Abstract: Tight regulation of apoptosis is essential for metazoan development and prevents diseases such as cancer and neurodegeneration. Caspase activation is central to apoptosis, and inhibitor of apoptosis ...Tight regulation of apoptosis is essential for metazoan development and prevents diseases such as cancer and neurodegeneration. Caspase activation is central to apoptosis, and inhibitor of apoptosis proteins (IAPs) are the principal actors that restrain caspase activity and are therefore attractive therapeutic targets. IAPs, in turn, are regulated by mitochondria-derived proapoptotic factors such as SMAC and HTRA2. Through a series of cryo-electron microscopy structures of full-length human baculoviral IAP repeat-containing protein 6 (BIRC6) bound to SMAC, caspases, and HTRA2, we provide a molecular understanding for BIRC6-mediated caspase inhibition and its release by SMAC. The architecture of BIRC6, together with near-irreversible binding of SMAC, elucidates how the IAP inhibitor SMAC can effectively control a processive ubiquitin ligase to respond to apoptotic stimuli. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_27841.map.gz emd_27841.map.gz | 157.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-27841-v30.xml emd-27841-v30.xml emd-27841.xml emd-27841.xml | 27.5 KB 27.5 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_27841_fsc.xml emd_27841_fsc.xml | 11.6 KB | Display |  FSC data file FSC data file |
| Images |  emd_27841.png emd_27841.png | 74.2 KB | ||
| Masks |  emd_27841_msk_1.map emd_27841_msk_1.map emd_27841_msk_2.map emd_27841_msk_2.map | 166.4 MB 52.7 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-27841.cif.gz emd-27841.cif.gz | 9.8 KB | ||
| Others |  emd_27841_half_map_1.map.gz emd_27841_half_map_1.map.gz emd_27841_half_map_2.map.gz emd_27841_half_map_2.map.gz | 154.6 MB 154.6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-27841 http://ftp.pdbj.org/pub/emdb/structures/EMD-27841 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-27841 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-27841 | HTTPS FTP |
-Validation report
| Summary document |  emd_27841_validation.pdf.gz emd_27841_validation.pdf.gz | 1.3 MB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_27841_full_validation.pdf.gz emd_27841_full_validation.pdf.gz | 1.3 MB | Display | |
| Data in XML |  emd_27841_validation.xml.gz emd_27841_validation.xml.gz | 20.5 KB | Display | |
| Data in CIF |  emd_27841_validation.cif.gz emd_27841_validation.cif.gz | 26.5 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-27841 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-27841 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-27841 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-27841 | HTTPS FTP |
-Related structure data
| Related structure data |  8e2kMC  8e2dC  8e2eC  8e2fC  8e2gC  8e2hC  8e2iC  8e2jC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_27841.map.gz / Format: CCP4 / Size: 166.4 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_27841.map.gz / Format: CCP4 / Size: 166.4 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | main map | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.2 Å | ||||||||||||||||||||||||||||||||||||
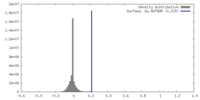
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_27841_msk_1.map emd_27841_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
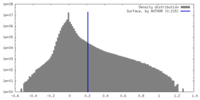
| Density Histograms |
-Mask #2
| File |  emd_27841_msk_2.map emd_27841_msk_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: half map 1
| File | emd_27841_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map 1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: half map 2
| File | emd_27841_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map 2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Baculoviral IAP repeat-containing protein 6
| Entire | Name: Baculoviral IAP repeat-containing protein 6 |
|---|---|
| Components |
|
-Supramolecule #1: Baculoviral IAP repeat-containing protein 6
| Supramolecule | Name: Baculoviral IAP repeat-containing protein 6 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 1.174 MDa |
-Macromolecule #1: Baculoviral IAP repeat-containing protein 6
| Macromolecule | Name: Baculoviral IAP repeat-containing protein 6 / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO / EC number: Ligases |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 535.317188 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MGDYKDHDGD YKDHDIDYKD DDDKGGGSGG LEVLFQGPSR TMVTGGGAAP PGTVTEPLPS VIVLSAGRKM AAAAAAASGP GCSSAAGAG AAGVSEWLVL RDGCMHCDAD GLHSLSYHPA LNAILAVTSR GTIKVIDGTS GATLQASALS AKPGGQVKCQ Y ISAVDKVI ...String: MGDYKDHDGD YKDHDIDYKD DDDKGGGSGG LEVLFQGPSR TMVTGGGAAP PGTVTEPLPS VIVLSAGRKM AAAAAAASGP GCSSAAGAG AAGVSEWLVL RDGCMHCDAD GLHSLSYHPA LNAILAVTSR GTIKVIDGTS GATLQASALS AKPGGQVKCQ Y ISAVDKVI FVDDYAVGCR KDLNGILLLD TALQTPVSKQ DDVVQLELPV TEAQQLLSAC LEKVDISSTE GYDLFITQLK DG LKNTSHE TAANHKVAKW ATVTFHLPHH VLKSIASAIV NELKKINQNV AALPVASSVM DRLSYLLPSA RPELGVGPGR SVD RSLMYS EANRRETFTS WPHVGYRWAQ PDPMAQAGFY HQPASSGDDR AMCFTCSVCL VCWEPTDEPW SEHERHSPNC PFVK GEHTQ NVPLSVTLAT SPAQFPCTDG TDRISCFGSG SCPHFLAAAT KRGKICIWDV SKLMKVHLKF EINAYDPAIV QQLIL SGDP SSGVDSRRPT LAWLEDSSSC SDIPKLEGDS DDLLEDSDSE EHSRSDSVTG HTSQKEAMEV SLDITALSIL QQPEKL QWE IVANVLEDTV KDLEELGANP CLTNSKSEKT KEKHQEQHNI PFPCLLAGGL LTYKSPATSP ISSNSHRSLD GLSRTQG ES ISEQGSTDNE SCTNSELNSP LVRRTLPVLL LYSIKESDEK AGKIFSQMNN IMSKSLHDDG FTVPQIIEME LDSQEQLL L QDPPVTYIQQ FADAAANLTS PDSEKWNSVF PKPGTLVQCL RLPKFAEEEN LCIDSITPCA DGIHLLVGLR TCPVESLSA INQVEALNNL NKLNSALCNR RKGELESNLA VVNGANISVI QHESPADVQT PLIIQPEQRN VSGGYLVLYK MNYATRIVTL EEEPIKIQH IKDPQDTITS LILLPPDILD NREDDCEEPI EDMQLTSKNG FEREKTSDIS TLGHLVITTQ GGYVKILDLS N FEILAKVE PPKKEGTEEQ DTFVSVIYCS GTDRLCACTK GGELHFLQIG GTCDDIDEAD ILVDGSLSKG IEPSSEGSKP LS NPSSPGI SGVDLLVDQP FTLEILTSLV ELTRFETLTP RFSATVPPCW VEVQQEQQQR RHPQHLHQQH HGDAAQHTRT WKL QTDSNS WDEHVFELVL PKACMVGHVD FKFVLNSNIT NIPQIQVTLL KNKAPGLGKV NALNIEVEQN GKPSLVDLNE EMQH MDVEE SQCLRLCPFL EDHKEDILCG PVWLASGLDL SGHAGMLTLT SPKLVKGMAG GKYRSFLIHV KAVNERGTEE ICNGG MRPV VRLPSLKHQS NKGYSLASLL AKVAAGKEKS SNVKNENTSG TRKSENLRGC DLLQEVSVTI RRFKKTSISK ERVQRC AML QFSEFHEKLV NTLCRKTDDG QITEHAQSLV LDTLCWLAGV HSNGPGSSKE GNENLLSKTR KFLSDIVRVC FFEAGRS IA HKCARFLALC ISNGKCDPCQ PAFGPVLLKA LLDNMSFLPA ATTGGSVYWY FVLLNYVKDE DLAGCSTACA SLLTAVSR Q LQDRLTPMEA LLQTRYGLYS SPFDPVLFDL EMSGSSCKNV YNSSIGVQSD EIDLSDVLSG NGKVSSCTAA EGSFTSLTG LLEVEPLHFT CVSTSDGTRI ERDDAMSSFG VTPAVGGLSS GTVGEASTAL SSAAQVALQS LSHAMASAEQ QLQVLQEKQQ QLLKLQQQK AKLEAKLHQT TAAAAAAASA VGPVHNSVPS NPVAAPGFFI HPSDVIPPTP KTTPLFMTPP LTPPNEAVSV V INAELAQL FPGSVIDPPA VNLAAHNKNS NKSRMNPLGS GLALAISHAS HFLQPPPHQS IIIERMHSGA RRFVTLDFGR PI LLTDVLI PTCGDLASLS IDIWTLGEEV DGRRLVVATD ISTHSLILHD LIPPPVCRFM KITVIGRYGS TNARAKIPLG FYY GHTYIL PWESELKLMH DPLKGEGESA NQPEIDQHLA MMVALQEDIQ CRYNLACHRL ETLLQSIDLP PLNSANNAQY FLRK PDKAV EEDSRVFSAY QDCIQLQLQL NLAHNAVQRL KVALGASRKM LSETSNPEDL IQTSSTEQLR TIIRYLLDTL LSLLH ASNG HSVPAVLQST FHAQACEELF KHLCISGTPK IRLHTGLLLV QLCGGERWWG QFLSNVLQEL YNSEQLLIFP QDRVFM LLS CIGQRSLSNS GVLESLLNLL DNLLSPLQPQ LPMHRRTEGV LDIPMISWVV MLVSRLLDYV ATVEDEAAAA KKPLNGN QW SFINNNLHTQ SLNRSSKGSS SLDRLYSRKI RKQLVHHKQQ LNLLKAKQKA LVEQMEKEKI QSNKGSSYKL LVEQAKLK Q ATSKHFKDLI RLRRTAEWSR SNLDTEVTTA KESPEIEPLP FTLAHERCIS VVQKLVLFLL SMDFTCHADL LLFVCKVLA RIANATRPTI HLCEIVNEPQ LERLLLLLVG TDFNRGDISW GGAWAQYSLT CMLQDILAGE LLAPVAAEAM EEGTVGDDVG ATAGDSDDS LQQSSVQLLE TIDEPLTHDI TGAPPLSSLE KDKEIDLELL QDLMEVDIDP LDIDLEKDPL AAKVFKPISS T WYDYWGAD YGTYNYNPYI GGLGIPVAKP PANTEKNGSQ TVSVSVSQAL DARLEVGLEQ QAELMLKMMS TLEADSILQA LT NTSPTLS QSPTGTDDSL LGGLQAANQT SQLIIQLSSV PMLNVCFNKL FSMLQVHHVQ LESLLQLWLT LSLNSSSTGN KEN GADIFL YNANRIPVIS LNQASITSFL TVLAWYPNTL LRTWCLVLHS LTLMTNMQLN SGSSSAIGTQ ESTAHLLVSD PNLI HVLVK FLSGTSPHGT NQHSPQVGPT ATQAMQEFLT RLQVHLSSTC PQIFSEFLLK LIHILSTERG AFQTGQGPLD AQVKL LEFT LEQNFEVVSV STISAVIESV TFLVHHYITC SDKVMSRSGS DSSVGARACF GGLFANLIRP GDAKAVCGEM TRDQLM FDL LKLVNILVQL PLSGNREYSA RVSVTTNTTD SVSDEEKVSG GKDGNGSSTS VQGSPAYVAD LVLANQQIMS QILSALG LC NSSAMAMIIG ASGLHLTKHE NFHGGLDAIS VGDGLFTILT TLSKKASTVH MMLQPILTYM ACGYMGRQGS LATCQLSE P LLWFILRVLD TSDALKAFHD MGGVQLICNN MVTSTRAIVN TARSMVSTIM KFLDSGPNKA VDSTLKTRIL ASEPDNAEG IHNFAPLGTI TSSSPTAQPA EVLLQATPPH RRARSAAWSY IFLPEEAWCD LTIHLPAAVL LKEIHIQPHL ASLATCPSSV SVEVSADGV NMLPLSTPVV TSGLTYIKIQ LVKAEVASAV CLRLHRPRDA STLGLSQIKL LGLTAFGTTS SATVNNPFLP S EDQVSKTS IGWLRLLHHC LTHISDLEGM MASAAAPTAN LLQTCAALLM SPYCGMHSPN IEVVLVKIGL QSTRIGLKLI DI LLRNCAA SGSDPTDLNS PLLFGRLNGL SSDSTIDILY QLGTTQDPGT KDRIQALLKW VSDSARVAAM KRSGRMNYMC PNS STVEYG LLMPSPSHLH CVAAILWHSY ELLVEYDLPA LLDQELFELL FNWSMSLPCN MVLKKAVDSL LCSMCHVHPN YFSL LMGWM GITPPPVQCH HRLSMTDDSK KQDLSSSLTD DSKNAQAPLA LTESHLATLA SSSQSPEAIK QLLDSGLPSL LVRSL ASFC FSHISSSESI AQSIDISQDK LRRHHVPQQC NKMPITADLV APILRFLTEV GNSHIMKDWL GGSEVNPLWT ALLFLL CHS GSTSGSHNLG AQQTSARSAS LSSAATTGLT TQQRTAIENA TVAFFLQCIS CHPNNQKLMA QVLCELFQTS PQRGNLP TS GNISGFIRRL FLQLMLEDEK VTMFLQSPCP LYKGRINATS HVIQHPMYGA GHKFRTLHLP VSTTLSDVLD RVSDTPSI T AKLISEQKDD KEKKNHEEKE KVKAENGFQD NYSVVVASGL KSQSKRAVSA TPPRPPSRRG RTIPDKIGST SGAEAANKI ITVPVFHLFH KLLAGQPLPA EMTLAQLLTL LYDRKLPQGY RSIDLTVKLG SRVITDPSLS KTDSYKRLHP EKDHGDLLAS CPEDEALTP GDECMDGILD ESLLETCPIQ SPLQVFAGMG GLALIAERLP MLYPEVIQQV SAPVVTSTTQ EKPKDSDQFE W VTIEQSGE LVYEAPETVA AEPPPIKSAV QTMSPIPAHS LAAFGLFLRL PGYAEVLLKE RKHAQCLLRL VLGVTDDGEG SH ILQSPSA NVLPTLPFHV LRSLFSTTPL TTDDGVLLRR MALEIGALHL ILVCLSALSH HSPRVPNSSV NQTEPQVSSS HNP TSTEEQ QLYWAKGTGF GTGSTASGWD VEQALTKQRL EEEHVTCLLQ VLASYINPVS SAVNGEAQSS HETRGQNSNA LPSV LLELL SQSCLIPAMS SYLRNDSVLD MARHVPLYRA LLELLRAIAS CAAMVPLLLP LSTENGEEEE EQSECQTSVG TLLAK MKTC VDTYTNRLRS KRENVKTGVK PDASDQEPEG LTLLVPDIQK TAEIVYAATT SLRQANQEKK LGEYSKKAAM KPKPLS VLK SLEEKYVAVM KKLQFDTFEM VSEDEDGKLG FKVNYHYMSQ VKNANDANSA ARARRLAQEA VTLSTSLPLS SSSSVFV RC DEERLDIMKV LITGPADTPY ANGCFEFDVY FPQDYPSSPP LVNLETTGGH SVRFNPNLYN DGKVCLSILN TWHGRPEE K WNPQTSSFLQ VLVSVQSLIL VAEPYFNEPG YERSRGTPSG TQSSREYDGN IRQATVKWAM LEQIRNPSPC FKEVIHKHF YLKRVEIMAQ CEEWIADIQQ YSSDKRVGRT MSHHAAALKR HTAQLREELL KLPCPEGLDP DTDDAPEVCR ATTGAEETLM HDQVKPSSS KELPSDFQL UniProtKB: Baculoviral IAP repeat-containing protein 6 |
-Macromolecule #2: Serine protease HTRA2, mitochondrial
| Macromolecule | Name: Serine protease HTRA2, mitochondrial / type: protein_or_peptide / ID: 2 / Number of copies: 3 / Enantiomer: LEVO / EC number: HtrA2 peptidase |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 35.961887 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MAVPSPPPAS PRSQYNFIAD VVEKTAPAVV YIEILDRHPF LGREVPISNG SGFVVAADGL IVTNAHVVAD RRRVRVRLLS GDTYEAVVT AVDPVADIAT LRIQTKEPLP TLPLGRSADV RQGEFVVAMG SPFALQNTIT SGIVSSAQRP ARDLGLPQTN V EYIQTDAA ...String: MAVPSPPPAS PRSQYNFIAD VVEKTAPAVV YIEILDRHPF LGREVPISNG SGFVVAADGL IVTNAHVVAD RRRVRVRLLS GDTYEAVVT AVDPVADIAT LRIQTKEPLP TLPLGRSADV RQGEFVVAMG SPFALQNTIT SGIVSSAQRP ARDLGLPQTN V EYIQTDAA IDFGNAGGPL VNLDGEVIGV NTMKVTAGIS FAIPSDRLRE FLHRGEKKNS SSGISGSQRR YIGVMMLTLS PS ILAELQL REPSFPDVQH GVLIHKVILG SPAHRAGLRP GDVILAIGEQ MVQNAEDVYE AVRTQSQLAV QIRRGRETLT LYV TPEVTE HHHHHH UniProtKB: Serine protease HTRA2, mitochondrial |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 1 mg/mL | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.4 Component:
| ||||||||||||
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Mesh: 300 / Support film - Material: CARBON / Support film - topology: HOLEY / Support film - Film thickness: 12 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 60 sec. / Pretreatment - Atmosphere: AIR / Pretreatment - Pressure: 0.039 kPa | ||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 90 % / Chamber temperature: 283.15 K / Instrument: LEICA EM GP | ||||||||||||
| Details | added CHAPSO to 0.4 mM |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Energy filter - Name: GIF Bioquantum / Energy filter - Slit width: 20 eV |
| Details | Data collection in counting mode, using multi-shot scheme (9 holes per stage position, 3 movies per hole) |
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Detector mode: COUNTING / Digitization - Dimensions - Width: 5760 pixel / Digitization - Dimensions - Height: 4092 pixel / Digitization - Frames/image: 1-50 / Number grids imaged: 1 / Number real images: 15309 / Average exposure time: 2.5 sec. / Average electron dose: 60.659 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.1 µm / Nominal defocus min: 0.8 µm / Nominal magnification: 105000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: OTHER |
|---|---|
| Output model |  PDB-8e2k: |
 Movie
Movie Controller
Controller
















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)