+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| タイトル | SPR reconstruction of AAV5 bound with PKD1-2 | |||||||||
 マップデータ マップデータ | SPR reconstruction of AAV5 bound with PKd1-2 | |||||||||
 試料 試料 |
| |||||||||
| 生物種 |  adeno-associated virus 5 (アデノ随伴ウイルス) / adeno-associated virus 5 (アデノ随伴ウイルス) /  Homo sapiens (ヒト) Homo sapiens (ヒト) | |||||||||
| 手法 | 単粒子再構成法 / クライオ電子顕微鏡法 / 解像度: 2.88 Å | |||||||||
 データ登録者 データ登録者 | Hu GQ / Silveria MA / Chapman MS / Stagg SM | |||||||||
| 資金援助 |  米国, 2件 米国, 2件
| |||||||||
 引用 引用 |  ジャーナル: J Virol / 年: 2022 ジャーナル: J Virol / 年: 2022タイトル: Adeno-Associated Virus Receptor-Binding: Flexible Domains and Alternative Conformations through Cryo-Electron Tomography of Adeno-Associated Virus 2 (AAV2) and AAV5 Complexes. 著者: Guiqing Hu / Mark A Silveria / Grant M Zane / Michael S Chapman / Scott M Stagg /  要旨: Recombinant forms of adeno-associated virus (rAAV) are vectors of choice in the development of treatments for a number of genetic dispositions. Greater understanding of AAV's molecular virology is ...Recombinant forms of adeno-associated virus (rAAV) are vectors of choice in the development of treatments for a number of genetic dispositions. Greater understanding of AAV's molecular virology is needed to underpin needed improvements in efficiency and specificity. Recent advances have included identification of a near-universal entry receptor, AAVR, and structures detected by cryo-electron microscopy (EM) single particle analysis (SPA) that revealed, at high resolution, only the domains of AAVR most tightly bound to AAV. Here, cryogenic electron tomography (cryo-ET) is applied to reveal the neighboring domains of the flexible receptor. For AAV5, where the PKD1 domain is bound strongly, PKD2 is seen in three configurations extending away from the virus. AAV2 binds tightly to the PKD2 domain at a distinct site, and cryo-ET now reveals four configurations of PKD1, all different from that seen in AAV5. The AAV2 receptor complex also shows unmodeled features on the inner surface that appear to be an equilibrium alternate configuration. Other AAV structures start near the 5-fold axis, but now β-strand A is the minor conformer and, for the major conformer, partially ordered N termini near the 2-fold axis join the canonical capsid jellyroll fold at the βA-βB turn. The addition of cryo-ET is revealing unappreciated complexity that is likely relevant to viral entry and to the development of improved gene therapy vectors. With 150 clinical trials for 30 diseases under way, AAV is a leading gene therapy vector. Immunotoxicity at high doses used to overcome inefficient transduction has occasionally proven fatal and highlighted gaps in fundamental virology. AAV enters cells, interacting through distinct sites with different domains of the AAVR receptor, according to AAV clade. Single domains are resolved in structures by cryogenic electron microscopy. Here, the adjoining domains are revealed by cryo-electron tomography of AAV2 and AAV5 complexes. They are in flexible configurations interacting minimally with AAV, despite measurable dependence of AAV2 transduction on both domains. | |||||||||
| 履歴 |
|
- 構造の表示
構造の表示
| 添付画像 |
|---|
- ダウンロードとリンク
ダウンロードとリンク
-EMDBアーカイブ
| マップデータ |  emd_26177.map.gz emd_26177.map.gz | 241 MB |  EMDBマップデータ形式 EMDBマップデータ形式 | |
|---|---|---|---|---|
| ヘッダ (付随情報) |  emd-26177-v30.xml emd-26177-v30.xml emd-26177.xml emd-26177.xml | 18.4 KB 18.4 KB | 表示 表示 |  EMDBヘッダ EMDBヘッダ |
| 画像 |  emd_26177.png emd_26177.png | 269.5 KB | ||
| その他 |  emd_26177_half_map_1.map.gz emd_26177_half_map_1.map.gz emd_26177_half_map_2.map.gz emd_26177_half_map_2.map.gz | 245 MB 244.8 MB | ||
| アーカイブディレクトリ |  http://ftp.pdbj.org/pub/emdb/structures/EMD-26177 http://ftp.pdbj.org/pub/emdb/structures/EMD-26177 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-26177 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-26177 | HTTPS FTP |
-検証レポート
| 文書・要旨 |  emd_26177_validation.pdf.gz emd_26177_validation.pdf.gz | 647.7 KB | 表示 |  EMDB検証レポート EMDB検証レポート |
|---|---|---|---|---|
| 文書・詳細版 |  emd_26177_full_validation.pdf.gz emd_26177_full_validation.pdf.gz | 647.3 KB | 表示 | |
| XML形式データ |  emd_26177_validation.xml.gz emd_26177_validation.xml.gz | 16.7 KB | 表示 | |
| CIF形式データ |  emd_26177_validation.cif.gz emd_26177_validation.cif.gz | 20 KB | 表示 | |
| アーカイブディレクトリ |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-26177 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-26177 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-26177 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-26177 | HTTPS FTP |
-関連構造データ
- リンク
リンク
| EMDBのページ |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- マップ
マップ
| ファイル |  ダウンロード / ファイル: emd_26177.map.gz / 形式: CCP4 / 大きさ: 307.5 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) ダウンロード / ファイル: emd_26177.map.gz / 形式: CCP4 / 大きさ: 307.5 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 注釈 | SPR reconstruction of AAV5 bound with PKd1-2 | ||||||||||||||||||||||||||||||||||||
| 投影像・断面図 | 画像のコントロール
画像は Spider により作成 | ||||||||||||||||||||||||||||||||||||
| ボクセルのサイズ | X=Y=Z: 1.084 Å | ||||||||||||||||||||||||||||||||||||
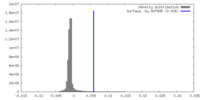
| 密度 |
| ||||||||||||||||||||||||||||||||||||
| 対称性 | 空間群: 1 | ||||||||||||||||||||||||||||||||||||
| 詳細 | EMDB XML:
|
-添付データ
-ハーフマップ: half map of SPR reconstruction of AAV5 bound with PKd1-2
| ファイル | emd_26177_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 注釈 | half map of SPR reconstruction of AAV5 bound with PKd1-2 | ||||||||||||
| 投影像・断面図 |
| ||||||||||||
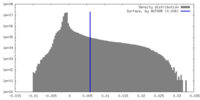
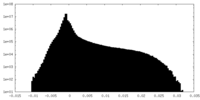
| 密度ヒストグラム |
-ハーフマップ: half map of SPR reconstruction of AAV5 bound with PKd1-2
| ファイル | emd_26177_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 注釈 | half map of SPR reconstruction of AAV5 bound with PKd1-2 | ||||||||||||
| 投影像・断面図 |
| ||||||||||||
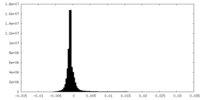
| 密度ヒストグラム |
- 試料の構成要素
試料の構成要素
-全体 : Binary complex of AAV-5 with a two domain fragment of its cellula...
| 全体 | 名称: Binary complex of AAV-5 with a two domain fragment of its cellular receptor, AAVR |
|---|---|
| 要素 |
|
-超分子 #1: Binary complex of AAV-5 with a two domain fragment of its cellula...
| 超分子 | 名称: Binary complex of AAV-5 with a two domain fragment of its cellular receptor, AAVR タイプ: complex / キメラ: Yes / ID: 1 / 親要素: 0 |
|---|---|
| 分子量 | 実験値: 82 KDa |
-超分子 #2: AAV-5
| 超分子 | 名称: AAV-5 / タイプ: complex / キメラ: Yes / ID: 2 / 親要素: 1 |
|---|---|
| 由来(天然) | 生物種:  adeno-associated virus 5 (アデノ随伴ウイルス) adeno-associated virus 5 (アデノ随伴ウイルス) |
| 組換発現 | 生物種:  |
| 分子量 | 理論値: 60 KDa |
-超分子 #3: AAVR
| 超分子 | 名称: AAVR / タイプ: complex / キメラ: Yes / ID: 3 / 親要素: 1 |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
| 組換発現 | 生物種:  |
| 分子量 | 理論値: 22 KDa |
-実験情報
-構造解析
| 手法 | クライオ電子顕微鏡法 |
|---|---|
 解析 解析 | 単粒子再構成法 |
| 試料の集合状態 | particle |
- 試料調製
試料調製
| 緩衝液 | pH: 7.4 構成要素:
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| グリッド | モデル: PELCO Ultrathin Carbon with Lacey Carbon / 材質: COPPER / メッシュ: 400 / 支持フィルム - 材質: CARBON / 支持フィルム - トポロジー: LACEY / 支持フィルム - Film thickness: 3.0 nm | |||||||||
| 凍結 | 凍結剤: ETHANE / チャンバー内湿度: 100 % / チャンバー内温度: 298 K / 装置: FEI VITROBOT MARK IV 詳細: 2 ul of 5.4 uM AAV5 VLP was added to the grid and given 2 minutes to adhere. Sample was then wicked and 2 uL of 33 uM PKD1-2 was added. Grids were then plunge frozen using an FEI Vitrobot ...詳細: 2 ul of 5.4 uM AAV5 VLP was added to the grid and given 2 minutes to adhere. Sample was then wicked and 2 uL of 33 uM PKD1-2 was added. Grids were then plunge frozen using an FEI Vitrobot Mark IV with a blot force of 4, time of 2 sec, temperature of 25C, and at 100 precent humidity.. | |||||||||
| 詳細 | 2 ul of 5.4 uM AAV5 VLP was added to the grid and given 2 minutes to adhere. Sample was then wicked and 2 uL of 33 uM PKD1-2 was added. Grids were then plunge frozen using an FEI Vitrobot Mark IV with a blot force of 4, time of 2 sec, temperature of 25C, and at 100 precent humidity. |
- 電子顕微鏡法
電子顕微鏡法
| 顕微鏡 | FEI TITAN KRIOS |
|---|---|
| 撮影 | フィルム・検出器のモデル: GATAN K3 (6k x 4k) / デジタル化 - サイズ - 横: 5760 pixel / デジタル化 - サイズ - 縦: 4092 pixel / 撮影したグリッド数: 1 / 実像数: 1200 / 平均露光時間: 2.9 sec. / 平均電子線量: 60.0 e/Å2 詳細: images were colleted in movie mode at 17 frames per second |
| 電子線 | 加速電圧: 300 kV / 電子線源:  FIELD EMISSION GUN FIELD EMISSION GUN |
| 電子光学系 | C2レンズ絞り径: 100.0 µm / 最大 デフォーカス(補正後): 3.0 µm / 最小 デフォーカス(補正後): 1.0 µm / 倍率(補正後): 83316 / 照射モード: FLOOD BEAM / 撮影モード: BRIGHT FIELD / Cs: 2.7 mm / 最大 デフォーカス(公称値): 3.0 µm / 最小 デフォーカス(公称値): 1.0 µm / 倍率(公称値): 81000 |
| 試料ステージ | 試料ホルダーモデル: FEI TITAN KRIOS AUTOGRID HOLDER ホルダー冷却材: NITROGEN |
| 実験機器 |  モデル: Titan Krios / 画像提供: FEI Company |
 ムービー
ムービー コントローラー
コントローラー













 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)