[English] 日本語
 Yorodumi
Yorodumi- EMDB-25719: Cryo-EM structure of TRPV5 in nanodiscs with PI(4,5)P2 at pH6 state 1 -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of TRPV5 in nanodiscs with PI(4,5)P2 at pH6 state 1 | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Calcium / Ion channel / Kidney / TRANSPORT PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationregulation of urine volume / calcium ion import across plasma membrane / calcium ion homeostasis / calcium ion transmembrane transport / calcium channel activity / calcium ion transport / protein homotetramerization / calmodulin binding / apical plasma membrane / identical protein binding ...regulation of urine volume / calcium ion import across plasma membrane / calcium ion homeostasis / calcium ion transmembrane transport / calcium channel activity / calcium ion transport / protein homotetramerization / calmodulin binding / apical plasma membrane / identical protein binding / metal ion binding / plasma membrane Similarity search - Function | |||||||||
| Biological species |  | |||||||||
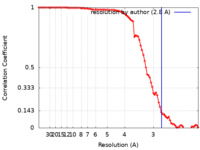
| Method | single particle reconstruction / cryo EM / Resolution: 2.8 Å | |||||||||
 Authors Authors | Fluck EC / Yazici AT | |||||||||
| Funding support |  United States, 2 items United States, 2 items
| |||||||||
 Citation Citation |  Journal: Cell Rep / Year: 2022 Journal: Cell Rep / Year: 2022Title: Structural basis of TRPV5 regulation by physiological and pathophysiological modulators. Authors: Edwin C Fluck / Aysenur Torun Yazici / Tibor Rohacs / Vera Y Moiseenkova-Bell /  Abstract: Transient receptor potential vanilloid 5 (TRPV5) is a kidney-specific Ca-selective ion channel that plays a key role in Ca homeostasis. The basal activity of TRPV5 is balanced through activation by ...Transient receptor potential vanilloid 5 (TRPV5) is a kidney-specific Ca-selective ion channel that plays a key role in Ca homeostasis. The basal activity of TRPV5 is balanced through activation by phosphatidylinositol 4,5-bisphosphate (PI(4,5)P) and inhibition by Ca-bound calmodulin (CaM). Parathyroid hormone (PTH), the key extrinsic regulator of Ca homeostasis, increases the activity of TRPV5 via protein kinase A (PKA)-mediated phosphorylation. Metabolic acidosis leads to reduced TRPV5 activity independent of PTH, causing hypercalciuria. Using cryoelectron microscopy (cryo-EM), we show that low pH inhibits TRPV5 by precluding PI(4,5)P activation. We capture intermediate conformations at low pH, revealing a transition from open to closed state. In addition, we demonstrate that PI(4,5)P is the primary modulator of channel gating, yet PKA controls TRPV5 activity by preventing CaM binding and channel inactivation. Our data provide detailed molecular mechanisms for regulation of TRPV5 by two key extrinsic modulators, low pH and PKA. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_25719.map.gz emd_25719.map.gz | 59.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-25719-v30.xml emd-25719-v30.xml emd-25719.xml emd-25719.xml | 15.2 KB 15.2 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_25719_fsc.xml emd_25719_fsc.xml | 8.9 KB | Display |  FSC data file FSC data file |
| Images |  emd_25719.png emd_25719.png | 126.8 KB | ||
| Filedesc metadata |  emd-25719.cif.gz emd-25719.cif.gz | 6.1 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-25719 http://ftp.pdbj.org/pub/emdb/structures/EMD-25719 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-25719 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-25719 | HTTPS FTP |
-Validation report
| Summary document |  emd_25719_validation.pdf.gz emd_25719_validation.pdf.gz | 562.3 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_25719_full_validation.pdf.gz emd_25719_full_validation.pdf.gz | 561.8 KB | Display | |
| Data in XML |  emd_25719_validation.xml.gz emd_25719_validation.xml.gz | 10.9 KB | Display | |
| Data in CIF |  emd_25719_validation.cif.gz emd_25719_validation.cif.gz | 14.2 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-25719 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-25719 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-25719 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-25719 | HTTPS FTP |
-Related structure data
| Related structure data |  7t6mMC  7t6jC  7t6kC  7t6lC  7t6nC  7t6oC  7t6pC  7t6qC  7t6rC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_25719.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_25719.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.06 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
- Sample components
Sample components
-Entire : Tetrameric assembly of TRPV5 ion channel reconstituted into lipid...
| Entire | Name: Tetrameric assembly of TRPV5 ion channel reconstituted into lipid nanodiscs |
|---|---|
| Components |
|
-Supramolecule #1: Tetrameric assembly of TRPV5 ion channel reconstituted into lipid...
| Supramolecule | Name: Tetrameric assembly of TRPV5 ion channel reconstituted into lipid nanodiscs type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Transient receptor potential cation channel subfamily V member 5
| Macromolecule | Name: Transient receptor potential cation channel subfamily V member 5 type: protein_or_peptide / ID: 1 / Details: Rabbit TRPV5 with c-terminal 1D4 epitope tag / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 83.784586 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MGACPPKAKG PWAQLQKLLI SWPVGEQDWE QYRDRVNMLQ QERIRDSPLL QAAKENDLRL LKILLLNQSC DFQQRGAVGE TALHVAALY DNLEAATLLM EAAPELAKEP ALCEPFVGQT ALHIAVMNQN LNLVRALLAR GASVSARATG AAFRRSPHNL I YYGEHPLS ...String: MGACPPKAKG PWAQLQKLLI SWPVGEQDWE QYRDRVNMLQ QERIRDSPLL QAAKENDLRL LKILLLNQSC DFQQRGAVGE TALHVAALY DNLEAATLLM EAAPELAKEP ALCEPFVGQT ALHIAVMNQN LNLVRALLAR GASVSARATG AAFRRSPHNL I YYGEHPLS FAACVGSEEI VRLLIEHGAD IRAQDSLGNT VLHILILQPN KTFACQMYNL LLSYDEHSDH LQSLELVPNH QG LTPFKLA GVEGNTVMFQ HLMQKRKHVQ WTCGPLTSTL YDLTEIDSWG EELSFLELVV SSKKREARQI LEQTPVKELV SFK WKKYGR PYFCVLASLY ILYMICFTTC CIYRPLKLRD DNRTDPRDIT ILQQKLLQEA YVTHQDNIRL VGELVTVTGA VIIL LLEIP DIFRVGASRY FGQTILGGPF HVIIITYASL VLLTMVMRLT NMNGEVVPLS FALVLGWCSV MYFARGFQML GPFTI MIQK MIFGDLMRFC WLMAVVILGF ASAFHITFQT EDPNNLGEFS DYPTALFSTF ELFLTIIDGP ANYSVDLPFM YCITYA AFA IIATLLMLNL FIAMMGDTHW RVAQERDELW RAQVVATTVM LERKMPRFLW PRSGICGYEY GLGDRWFLRV ENHHDQN PL RVLRYVEAFK CSDKEDGQEQ LSEKRPSTVE SGMLSRASVA FQTPSLSRTT SQSSNSHRGW EILRRNTLGH LNLGLDLG E GDGEEVYHFT ETSQVAPA UniProtKB: Transient receptor potential cation channel subfamily V member 5 |
-Macromolecule #2: [(2R)-2-octanoyloxy-3-[oxidanyl-[(1R,2R,3S,4R,5R,6S)-2,3,6-tris(o...
| Macromolecule | Name: [(2R)-2-octanoyloxy-3-[oxidanyl-[(1R,2R,3S,4R,5R,6S)-2,3,6-tris(oxidanyl)-4,5-diphosphonooxy-cyclohexyl]oxy-phosphoryl]oxy-propyl] octanoate type: ligand / ID: 2 / Number of copies: 4 / Formula: PIO |
|---|---|
| Molecular weight | Theoretical: 746.566 Da |
| Chemical component information |  ChemComp-PIO: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 6 |
|---|---|
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Mesh: 300 / Support film - Material: CARBON / Support film - topology: HOLEY / Support film - Film thickness: 12 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 30 sec. |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277.15 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Number grids imaged: 1 / Number real images: 7006 / Average electron dose: 42.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.5 µm / Nominal defocus min: 0.5 µm / Nominal magnification: 81000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL |
|---|---|
| Output model |  PDB-7t6m: |
 Movie
Movie Controller
Controller














 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)