[English] 日本語
 Yorodumi
Yorodumi- EMDB-20086: In situ structure of rotavirus VP1 RNA-dependent RNA polymerase (TLP) -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-20086 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | In situ structure of rotavirus VP1 RNA-dependent RNA polymerase (TLP) | |||||||||
 Map data Map data | filtered, B-sharpened, masked | |||||||||
 Sample Sample | Rhesus rotavirus != Rotavirus A (strain RVA/Monkey/United States/RRV/1975/G3P5B[3]) Rhesus rotavirus
| |||||||||
 Keywords Keywords | Rotavirus / RNA-dependent RNA polymerase / VP1 / VP2 / VIRAL PROTEIN-TRANSFERASE complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationT=2 icosahedral viral capsid / viral inner capsid / viral genome replication / virion component / viral nucleocapsid / RNA-directed RNA polymerase / nucleotide binding / RNA-directed RNA polymerase activity / DNA-templated transcription / RNA binding Similarity search - Function | |||||||||
| Biological species |  Rotavirus A (strain RVA/Monkey/United States/RRV/1975/G3P5B[3]) Rotavirus A (strain RVA/Monkey/United States/RRV/1975/G3P5B[3]) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 4.5 Å | |||||||||
 Authors Authors | Jenni S / Salgado EN | |||||||||
| Funding support |  United States, 2 items United States, 2 items
| |||||||||
 Citation Citation |  Journal: J Mol Biol / Year: 2019 Journal: J Mol Biol / Year: 2019Title: In situ Structure of Rotavirus VP1 RNA-Dependent RNA Polymerase. Authors: Simon Jenni / Eric N Salgado / Tobias Herrmann / Zongli Li / Timothy Grant / Nikolaus Grigorieff / Stefano Trapani / Leandro F Estrozi / Stephen C Harrison /   Abstract: Rotaviruses, like other non-enveloped, double-strand RNA viruses, package an RNA-dependent RNA polymerase (RdRp) with each duplex of their segmented genomes. Rotavirus cell entry results in loss of ...Rotaviruses, like other non-enveloped, double-strand RNA viruses, package an RNA-dependent RNA polymerase (RdRp) with each duplex of their segmented genomes. Rotavirus cell entry results in loss of an outer protein layer and delivery into the cytosol of an intact, inner capsid particle (the "double-layer particle," or DLP). The RdRp, designated VP1, is active inside the DLP; each VP1 achieves many rounds of mRNA transcription from its associated genome segment. Previous work has shown that one VP1 molecule lies close to each 5-fold axis of the icosahedrally symmetric DLP, just beneath the inner surface of its protein shell, embedded in tightly packed RNA. We have determined a high-resolution structure for the rotavirus VP1 RdRp in situ, by local reconstruction of density around individual 5-fold positions. We have analyzed intact virions ("triple-layer particles"), non-transcribing DLPs and transcribing DLPs. Outer layer dissociation enables the DLP to synthesize RNA, in vitro as well as in vivo, but appears not to induce any detectable structural change in the RdRp. Addition of NTPs, Mg, and S-adenosylmethionine, which allows active transcription, results in conformational rearrangements, in both VP1 and the DLP capsid shell protein, that allow a transcript to exit the polymerase and the particle. The position of VP1 (among the five symmetrically related alternatives) at one vertex does not correlate with its position at other vertices. This stochastic distribution of site occupancies limits long-range order in the 11-segment, double-strand RNA genome. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_20086.map.gz emd_20086.map.gz | 9.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-20086-v30.xml emd-20086-v30.xml emd-20086.xml emd-20086.xml | 22.3 KB 22.3 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_20086_fsc.xml emd_20086_fsc.xml | 12.5 KB | Display |  FSC data file FSC data file |
| Images |  emd_20086.png emd_20086.png | 144.8 KB | ||
| Masks |  emd_20086_msk_1.map emd_20086_msk_1.map | 103 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-20086.cif.gz emd-20086.cif.gz | 6.9 KB | ||
| Others |  emd_20086_additional_1.map.gz emd_20086_additional_1.map.gz emd_20086_additional_2.map.gz emd_20086_additional_2.map.gz emd_20086_half_map_1.map.gz emd_20086_half_map_1.map.gz emd_20086_half_map_2.map.gz emd_20086_half_map_2.map.gz | 94.4 MB 45.9 MB 9.4 MB 9.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-20086 http://ftp.pdbj.org/pub/emdb/structures/EMD-20086 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-20086 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-20086 | HTTPS FTP |
-Related structure data
| Related structure data |  6oj3MC  6oj4C  6oj5C  6oj6C C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_20086.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_20086.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | filtered, B-sharpened, masked | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
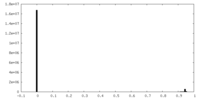
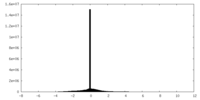
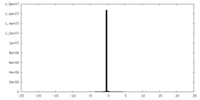
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.23 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
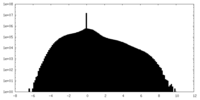
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_20086_msk_1.map emd_20086_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
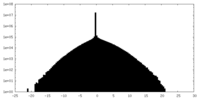
| Density Histograms |
-Additional map: filtered, B-sharpened
| File | emd_20086_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | filtered, B-sharpened | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: filtered
| File | emd_20086_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | filtered | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: half-map 1, unmodified
| File | emd_20086_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half-map 1, unmodified | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: half-map 2, unmodified
| File | emd_20086_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half-map 2, unmodified | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Rhesus rotavirus
| Entire | Name:  Rhesus rotavirus Rhesus rotavirus |
|---|---|
| Components |
|
-Supramolecule #1: Rotavirus A (strain RVA/Monkey/United States/RRV/1975/G3P5B[3])
| Supramolecule | Name: Rotavirus A (strain RVA/Monkey/United States/RRV/1975/G3P5B[3]) type: virus / ID: 1 / Parent: 0 / Macromolecule list: all / Details: TLP / NCBI-ID: 444185 Sci species name: Rotavirus A (strain RVA/Monkey/United States/RRV/1975/G3P5B[3]) Sci species strain: RVA/Monkey/United States/RRV/1975/G3P5B[3] Virus type: VIRION / Virus isolate: SEROTYPE / Virus enveloped: No / Virus empty: No |
|---|
-Macromolecule #1: Inner capsid protein VP2
| Macromolecule | Name: Inner capsid protein VP2 / type: protein_or_peptide / ID: 1 / Number of copies: 10 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Rotavirus A (strain RVA/Monkey/United States/RRV/1975/G3P5B[3]) Rotavirus A (strain RVA/Monkey/United States/RRV/1975/G3P5B[3])Strain: RVA/Monkey/United States/RRV/1975/G3P5B[3] |
| Molecular weight | Theoretical: 103.425992 KDa |
| Sequence | String: MAYRKRGARR ETNLKQDDRM QEKEENKNVN TNSENKNATK PQLSEKVLSQ KEEVITDNQE EIKIADEVKK SNKEESKQLL EVLKTKEEH QKEVQYEILQ KTIPTFEPKE SILKKLEDIK PEQVKKQTKL FRIFEPRQLP VYRANGEKEL RNRWYWKLKR D TLPDGDYD ...String: MAYRKRGARR ETNLKQDDRM QEKEENKNVN TNSENKNATK PQLSEKVLSQ KEEVITDNQE EIKIADEVKK SNKEESKQLL EVLKTKEEH QKEVQYEILQ KTIPTFEPKE SILKKLEDIK PEQVKKQTKL FRIFEPRQLP VYRANGEKEL RNRWYWKLKR D TLPDGDYD VREYFLNLYD QVLTEMPDYL LLKDMAVENK NSRDAGKVVD SETAAICDAI FQDEETEGVV RRFIAEMRQR VQ ADRNVVN YPSILHPIDH AFNEYFLQHQ LVEPLNNDII FNYIPERIRN DVNYILNMDR NLPSTARYIR PNLLQDRLNL HDN FESLWD TITTSNYILA RSVVPDLKEL VSTEAQIQKM SQDLQLEALT IQSETQFLTG INSQAANDCF KTLIAAMLSQ RTMS LDFVT TNYMSLISGM WLLTVVPNDM FIRESLVACQ LAIINTIIYP AFGMQRMHYR NGDPQTPFQI AEQQIQNFQV ANWLH FVNN NQFRQVVIDG VLNQVLNDNI RNGHVVNQLM EALMQLSRQQ FPTMPVDYKR SIQRGILLLS NRLGQLVDLT RLLAYN YET LMACITMNMQ HVQTLTTEKL QLTSVTSLCM LIGNATVIPS PQTLFHYYNV NVNFHSNYNE RINDAVAIIT AANRLNL YQ KKMKSIVEDF LKRLQIFDIS RVPDDQMYRL RDRLRLLPVE IRRLDIFNLI LMNMEQIERA SDKIAQGVII AYRDMQLE R DEMYGYVNIA RNLDGFQQIN LEELMRTGDY AQITNMLLNN QPVALVGALP FITDSSVISL VAKLDATVFA QIVKLRKVD TLKPILYKIN SDSNDFYLVA NYDWVPTSTT KVYKQIPQQF DFRASMHMLT SNLTFTVYSD LLAFVSADTV EPINAVAFDN MRIMNEL UniProtKB: Inner capsid protein VP2 |
-Macromolecule #2: RNA-directed RNA polymerase
| Macromolecule | Name: RNA-directed RNA polymerase / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO / EC number: RNA-directed RNA polymerase |
|---|---|
| Source (natural) | Organism:  Rotavirus A (strain RVA/Monkey/United States/RRV/1975/G3P5B[3]) Rotavirus A (strain RVA/Monkey/United States/RRV/1975/G3P5B[3])Strain: RVA/Monkey/United States/RRV/1975/G3P5B[3] |
| Molecular weight | Theoretical: 125.276305 KDa |
| Sequence | String: MGKYNLILSE YLSFIYNSQS AVQIPIYYSS NSELENRCIE FHSKCLENSK NGLSLKKLFV EYSDVIENAT LLSILSYSYD KYNAVERKL VKYAKGKPLE ADLTVNELDY ENNKITSELF PTAEEYTDLL MDPAILTSLS SNLNAVMFWL EKHENDVAEK L KIYKRRLD ...String: MGKYNLILSE YLSFIYNSQS AVQIPIYYSS NSELENRCIE FHSKCLENSK NGLSLKKLFV EYSDVIENAT LLSILSYSYD KYNAVERKL VKYAKGKPLE ADLTVNELDY ENNKITSELF PTAEEYTDLL MDPAILTSLS SNLNAVMFWL EKHENDVAEK L KIYKRRLD LFTIVASTVN KYGVPRHNAK YRYEYEVMKD KPYYLVTWAN SSIEMLMSVF SHEDYLIARE LIVLSYSNRS TL AKLVSSP MSILVALVDI NGTFITNEEL ELEFSNKYVR AIVPDQTFDE LKQMLDNMRK AGLTDIPKMI QDWLVDCSIE KFP LMAKIY SWSFHVGFRK QKMLDAALDQ LKTEYTEDVD DEMYREYTML IRDEVVKMLE EPVKHDDHLL QDSELAGLLS MSSA SNGES RQLKFGRKTI FSTKKNMHVM DDMANGRYTP GIIPPVNVDK PIPLGRRDVP GRRTRIIFIL PYEYFIAQHA VVEKM LIYA KHTREYAEFY SQSNQLLSYG DVTRFLSNNS MVLYTDVSQW DSSQHNTQPF RKGIIMGLDM LANMTNDARV IQTLNL YKQ TQINLMDSYV QIPDGNVIKK IQYGAVASGE KQTKAANSIA NLALIKTVLS RISNKYSFAT KIIRVDGDDN YAVLQFN TE VTKQMVQDVS NDVRETYARM NTKVKALVST VGIEIAKRYI AGGKIFFRAG INLLNNEKKG QSTQWDQAAV LYSNYIVN R LRGFETDREF ILTKIMQMTS VAITGSLRLF PSERVLTTNS TFKVFDSEDF IIEYGTTDDE VYIQRAFMSL SSQKSGIAD EIAASSTFKN YVSRLSEQLL FSKNNIVSRG IALTEKAKLN SYAPISLEKR RAQISALLTM LQKPVTFKSS KITINDILRD IKPFFTVNE AHLPIQYQKF MPTLPDNVQY IIQCIGSRTY QIEDDGSKSA ISRLISKYSV YKPSIEELYK VISLHENEIQ L YLISLGIP KIDADTYVGS KIYSQDKYRI LESYVYNLLS INYGCYQLFD FNSPDLEKLI RIPFKGKIPA VTFILHLYAK LE VINHAIK NGSWISLFCN YPKSEMIKLW KKMWNITSLR SPYTNANFFQ D UniProtKB: RNA-directed RNA polymerase |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Grid | Details: unspecified |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI POLARA 300 |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 64.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Tecnai Polara / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Protocol: AB INITIO MODEL |
|---|---|
| Output model |  PDB-6oj3: |
 Movie
Movie Controller
Controller














 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)