[English] 日本語
 Yorodumi
Yorodumi- PDB-1ivd: STRUCTURES OF AROMATIC INHIBITORS OF INFLUENZA VIRUS NEURAMINIDASE -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1ivd | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | STRUCTURES OF AROMATIC INHIBITORS OF INFLUENZA VIRUS NEURAMINIDASE | |||||||||
 Components Components | INFLUENZA A SUBTYPE N2 NEURAMINIDASE | |||||||||
 Keywords Keywords | HYDROLASE (O-GLYCOSYL) | |||||||||
| Function / homology |  Function and homology information Function and homology informationexo-alpha-sialidase / exo-alpha-sialidase activity / viral budding from plasma membrane / carbohydrate metabolic process / host cell plasma membrane / virion membrane / metal ion binding / membrane Similarity search - Function | |||||||||
| Biological species |   Influenza A virus Influenza A virus | |||||||||
| Method |  X-RAY DIFFRACTION / Resolution: 1.9 Å X-RAY DIFFRACTION / Resolution: 1.9 Å | |||||||||
 Authors Authors | Jedrzejas, M.J. / Luo, M. | |||||||||
 Citation Citation |  Journal: Biochemistry / Year: 1995 Journal: Biochemistry / Year: 1995Title: Structures of aromatic inhibitors of influenza virus neuraminidase. Authors: Jedrzejas, M.J. / Singh, S. / Brouillette, W.J. / Laver, W.G. / Air, G.M. / Luo, M. #1:  Journal: To be Published Journal: To be PublishedTitle: Structure-Based Inhibitors of Influenza Viral Neuraminidase. A Benzoic Acid Lead with Novel Interaction Authors: Singh, S. / Jedrzejas, M.J. / Singh, S. / Air, G.M. / Luo, M. / Laver, W.G. / Brouillette, W.J. #2:  Journal: To be Published Journal: To be PublishedTitle: Benzoic Acid Inhibitors of Influenza Virus Neuraminidase Authors: Luo, M. / Jedrzejas, M.J. / Singh, S. / White, C.L. / Brouillette, W.J. / Air, G.M. / Laver, W.G. #3:  Journal: J.Mol.Biol. / Year: 1991 Journal: J.Mol.Biol. / Year: 1991Title: Three-Dimensional Structure of the Neuraminidase of Influenza Virus A(Slash)Tokyo(Slash)3(Slash)67 at 2.2 Angstroms Resolution Authors: Varghese, J.N. / Colman, P.M. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1ivd.cif.gz 1ivd.cif.gz | 213.9 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1ivd.ent.gz pdb1ivd.ent.gz | 173.2 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1ivd.json.gz 1ivd.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/iv/1ivd https://data.pdbj.org/pub/pdb/validation_reports/iv/1ivd ftp://data.pdbj.org/pub/pdb/validation_reports/iv/1ivd ftp://data.pdbj.org/pub/pdb/validation_reports/iv/1ivd | HTTPS FTP |
|---|
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| Unit cell |
| ||||||||
| Atom site foot note | 1: CIS PROLINE - PRO 285 / 2: CIS PROLINE - PRO 326 | ||||||||
| Noncrystallographic symmetry (NCS) | NCS oper: (Code: given / Matrix: (1),| Details | MTRIX THE TRANSFORMATIONS PRESENTED ON MTRIX RECORDS BELOW DESCRIBE NON-CRYSTALLOGRAPHIC RELATIONSHIPS AMONG THE VARIOUS DOMAINS IN THIS ENTRY. APPLYING THE APPROPRIATE MTRIX TRANSFORMATION TO THE RESIDUES LISTED FIRST WILL YIELD APPROXIMATE COORDINATES FOR THE RESIDUES LISTED SECOND. APPLIED TO TRANSFORMED TO MTRIX RESIDUES RESIDUES RMSD M1 82 .. 469 .. | |
- Components
Components
-Protein , 1 types, 2 molecules AB
| #1: Protein | Mass: 43141.012 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Influenza A virus (strain A/Tokyo/3/1967 H2N2) Influenza A virus (strain A/Tokyo/3/1967 H2N2)Strain: A/Tokyo/3/1967 H2N2 / References: UniProt: P06820, exo-alpha-sialidase |
|---|
-Sugars , 4 types, 8 molecules
| #2: Polysaccharide | 2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose Source method: isolated from a genetically manipulated source #3: Polysaccharide | beta-D-mannopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-[beta-L-fucopyranose-(1-6) ...beta-D-mannopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-[beta-L-fucopyranose-(1-6)]2-acetamido-2-deoxy-beta-D-glucopyranose | Source method: isolated from a genetically manipulated source #4: Polysaccharide | Source method: isolated from a genetically manipulated source #5: Polysaccharide | beta-D-mannopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-[alpha-L-fucopyranose-(1- ...beta-D-mannopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-[alpha-L-fucopyranose-(1-6)]2-acetamido-2-deoxy-beta-D-glucopyranose | Source method: isolated from a genetically manipulated source |
|---|
-Non-polymers , 3 types, 129 molecules 
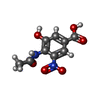



| #6: Chemical | | #7: Chemical | #8: Water | ChemComp-HOH / | |
|---|
-Details
| Has protein modification | Y |
|---|---|
| Nonpolymer details | THE CALCIUM, CA 470, STABILIZES A LOOP NEAR THE NEURAMINIDASE ACTIVE SITE. THE BANA105 INHIBITOR IS ...THE CALCIUM, CA 470, STABILIZES |
| Source details | MOLECULE_NAME: BANA105 SYNTHETIC. SEE SINGH ET AL. (SUBMITTED TO J. MED CHEM.) AND JEDRZEJAS ET AL. ...MOLECULE_NAME: BANA105 SYNTHETIC. SEE SINGH ET AL. (SUBMITTED TO J. MED CHEM.) AND JEDRZEJAS ET AL. (ACCEPTED BY BIOCHEMIST |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION X-RAY DIFFRACTION |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.42 Å3/Da / Density % sol: 63.99 % |
|---|---|
| Crystal grow | Details: NATIVE CRYSTALS SOAKED IN 5MM BANA105 SOLUTION, PH 6.8. |
| Crystal | *PLUS Density % sol: 58 % |
| Crystal grow | *PLUS Method: vapor diffusion, hanging drop |
-Data collection
| Radiation | Scattering type: x-ray |
|---|---|
| Radiation wavelength | Relative weight: 1 |
| Reflection | Num. obs: 33264 / Observed criterion σ(I): 1 |
| Reflection | *PLUS Rmerge(I) obs: 0.119 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Resolution: 1.9→5 Å / σ(F): 4 Details: ONLY HALF OF THE ASYMMETRIC UNIT WAS REFINED; THE REMAINING HALF WAS GENERATED USING A NONCRYSTALLOGRAPHIC TWO-FOLD AXIS. THE TOPOLOGY AND PARAMETER VALUES GENERATED FOR THE BANA105 RESIDUES ...Details: ONLY HALF OF THE ASYMMETRIC UNIT WAS REFINED; THE REMAINING HALF WAS GENERATED USING A NONCRYSTALLOGRAPHIC TWO-FOLD AXIS. THE TOPOLOGY AND PARAMETER VALUES GENERATED FOR THE BANA105 RESIDUES WERE EITHER TAKEN DIRECTLY FROM THE LITERATURE OR BY COMPARISON TO OTHER SIMILAR STRUCTURES IN THE X-PLOR TOPOLOGY AND PARAMETER LIBRARY FILES. MORE INFORMATION CONCERNING THE REFINEMENT PROTOCOLS AND BANA105 FILES IS PRESENTED IN THE ORIGINATING PAPER.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.9→5 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller
















 PDBj
PDBj



