[English] 日本語
 Yorodumi
Yorodumi- PDB-1au2: CRYSTAL STRUCTURE OF THE CYSTEINE PROTEASE HUMAN CATHEPSIN K IN C... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1au2 | ||||||
|---|---|---|---|---|---|---|---|
| Title | CRYSTAL STRUCTURE OF THE CYSTEINE PROTEASE HUMAN CATHEPSIN K IN COMPLEX WITH A COVALENT PROPANONE INHIBITOR | ||||||
 Components Components | CATHEPSIN K | ||||||
 Keywords Keywords | HYDROLASE / SULFHYDRYL PROTEINASE | ||||||
| Function / homology |  Function and homology information Function and homology informationcathepsin K / negative regulation of cartilage development / RUNX1 regulates transcription of genes involved in differentiation of keratinocytes / endolysosome lumen / thyroid hormone generation / Trafficking and processing of endosomal TLR / proteoglycan binding / Activation of Matrix Metalloproteinases / Collagen degradation / collagen catabolic process ...cathepsin K / negative regulation of cartilage development / RUNX1 regulates transcription of genes involved in differentiation of keratinocytes / endolysosome lumen / thyroid hormone generation / Trafficking and processing of endosomal TLR / proteoglycan binding / Activation of Matrix Metalloproteinases / Collagen degradation / collagen catabolic process / fibronectin binding / extracellular matrix disassembly / bone resorption / mitophagy / collagen binding / Degradation of the extracellular matrix / MHC class II antigen presentation / cysteine-type peptidase activity / lysosomal lumen / : / lysosome / apical plasma membrane / external side of plasma membrane / serine-type endopeptidase activity / cysteine-type endopeptidase activity / intracellular membrane-bounded organelle / proteolysis / extracellular space / extracellular region / nucleoplasm Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  molecular replacement / Resolution: 2.6 Å molecular replacement / Resolution: 2.6 Å | ||||||
 Authors Authors | Zhao, B. / Smith, W.W. / Janson, C.A. / Abdel-Meguid, S.S. | ||||||
 Citation Citation | Journal: J.Am.Chem.Soc. / Year: 1997 Title: Structure and Design of Potent and Selective Cathepsin K Inhibitors Authors: Yamashita, D.S. / Smith, W.W. / Zhao, B. / Janson, C.A. / Tomaszek, T.A. / Bossard, M.J. / Levy, M.A. / Oh, H.-J. / Carr, T.J. / Thompson, S.K. / Ijames, C.F. / Carr, S.A. / Mcqueney, M. / ...Authors: Yamashita, D.S. / Smith, W.W. / Zhao, B. / Janson, C.A. / Tomaszek, T.A. / Bossard, M.J. / Levy, M.A. / Oh, H.-J. / Carr, T.J. / Thompson, S.K. / Ijames, C.F. / Carr, S.A. / Mcqueney, M. / D'Alessio, K.J. / Amegadzie, B.Y. / Hanning, C.R. / Abdel-Meguid, S. / Desjarlais, R.L. / Gleason, J.G. / Veber, D.F. | ||||||
| History |
|
- Structure visualization
Structure visualization
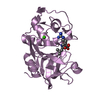
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1au2.cif.gz 1au2.cif.gz | 52.1 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1au2.ent.gz pdb1au2.ent.gz | 35.6 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1au2.json.gz 1au2.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/au/1au2 https://data.pdbj.org/pub/pdb/validation_reports/au/1au2 ftp://data.pdbj.org/pub/pdb/validation_reports/au/1au2 ftp://data.pdbj.org/pub/pdb/validation_reports/au/1au2 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  1atkS S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 23523.480 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Details: INHIBITOR COVALENTLY BOUND TO ACTIVE SITE CYS 25 / Source: (gene. exp.)  Homo sapiens (human) / Cell: OSTEOCLAST / Cell line: SF9 / Cell line (production host): SF9 / Production host: Homo sapiens (human) / Cell: OSTEOCLAST / Cell line: SF9 / Cell line (production host): SF9 / Production host:  |
|---|---|
| #2: Chemical | ChemComp-POS / |
| #3: Water | ChemComp-HOH / |
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.38 Å3/Da / Density % sol: 48 % | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | pH: 4.5 Details: 18% PEG 8000, 0.12M LI2SO4, 0.06M SODIUM ACETATE, PH 4.5 | ||||||||||||||||||||||||
| Crystal grow | *PLUS Method: vapor diffusion | ||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction | Mean temperature: 293 K |
|---|---|
| Diffraction source | Source:  ROTATING ANODE / Type: SIEMENS / Wavelength: 1.5418 ROTATING ANODE / Type: SIEMENS / Wavelength: 1.5418 |
| Detector | Type: SIEMENS / Detector: AREA DETECTOR / Date: Oct 1, 1996 / Details: COLLIMATOR |
| Radiation | Monochromator: GRAPHITE(002) / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.5418 Å / Relative weight: 1 |
| Reflection | Resolution: 2.4→25 Å / Num. obs: 34271 / % possible obs: 92 % / Observed criterion σ(I): 2 / Redundancy: 4 % / Rmerge(I) obs: 0.092 / Rsym value: 0.092 / Net I/σ(I): 6.3 |
| Reflection shell | Resolution: 2.4→2.59 Å / Redundancy: 3.5 % / Rmerge(I) obs: 0.32 / Mean I/σ(I) obs: 1.6 / Rsym value: 0.42 / % possible all: 74 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  molecular replacement molecular replacementStarting model: 1ATK Resolution: 2.6→8 Å / Rfactor Rfree error: 0.013 / Data cutoff high absF: 1000000 / Data cutoff low absF: 0.001 / Isotropic thermal model: OVERALL / Cross valid method: THROUGHOUT / σ(F): 2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 15 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine analyze |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.6→8 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 2.6→2.71 Å / Rfactor Rfree error: 0.076 / Total num. of bins used: 8
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Xplor file |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Software | *PLUS Name:  X-PLOR / Version: 3.1 / Classification: refinement X-PLOR / Version: 3.1 / Classification: refinement | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement | *PLUS σ(F): 2 / Rfactor obs: 0.24 / Rfactor Rwork: 0.24 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints | *PLUS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | *PLUS Rfactor Rfree: 0.46 |
 Movie
Movie Controller
Controller











 PDBj
PDBj